Answered step by step
Verified Expert Solution
Question
1 Approved Answer
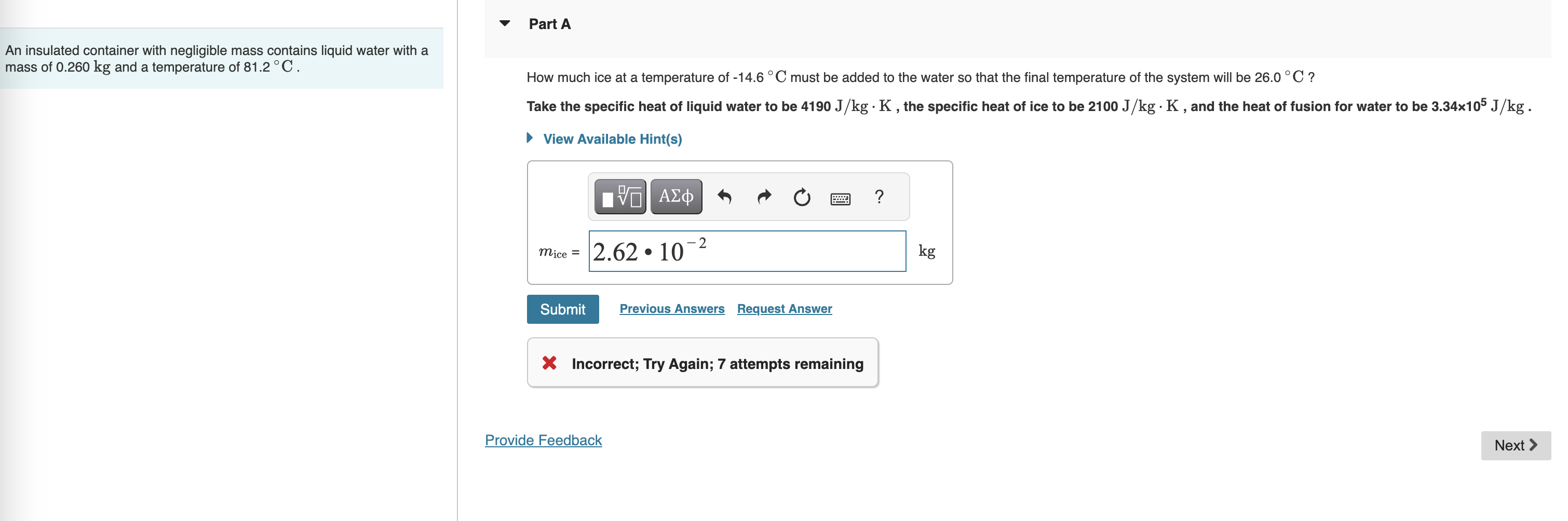
Part A An insulated container with negligible mass contains liquid water with a mass of 0 . 2 6 0 k g and a temperature
Part A
An insulated container with negligible mass contains liquid water with a
mass of and a temperature of
How much ice at a temperature of must be added to the water so that the final temperature of the system will be
Take the specific heat of liquid water to be the specific heat of ice to be and the heat of fusion for water to be
View Available Hints
Request Answer
Incorrect; Try Again; attempts remaining
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


