Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part a: CO adsorption on a Ni catalyst follows the Langmuir adsorption isotherm. Given the following data determine the Langmuir adsorption constant for CO
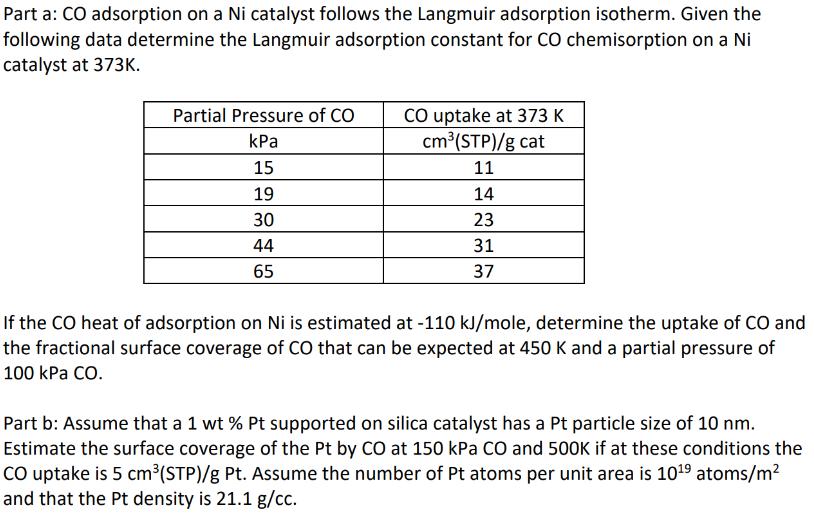
Part a: CO adsorption on a Ni catalyst follows the Langmuir adsorption isotherm. Given the following data determine the Langmuir adsorption constant for CO chemisorption on a Ni catalyst at 373K. Partial Pressure of CO kPa 15 19 30 44 65 CO uptake at 373 K cm (STP)/g cat 11 14 23 31 37 If the CO heat of adsorption on Ni is estimated at -110 kJ/mole, determine the uptake of CO and the fractional surface coverage of CO that can be expected at 450 K and a partial pressure of 100 kPa CO. Part b: Assume that a 1 wt% Pt supported on silica catalyst has a Pt particle size of 10 nm. Estimate the surface coverage of the Pt by CO at 150 kPa CO and 500K if at these conditions the CO uptake is 5 cm(STP)/g Pt. Assume the number of Pt atoms per unit area is 1019 atoms/m and that the Pt density is 21.1 g/cc.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


