Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part A Describe the hybridization of the carbon atom in the poisonous gas phosgene, Cl CO. Match the words in the left column to
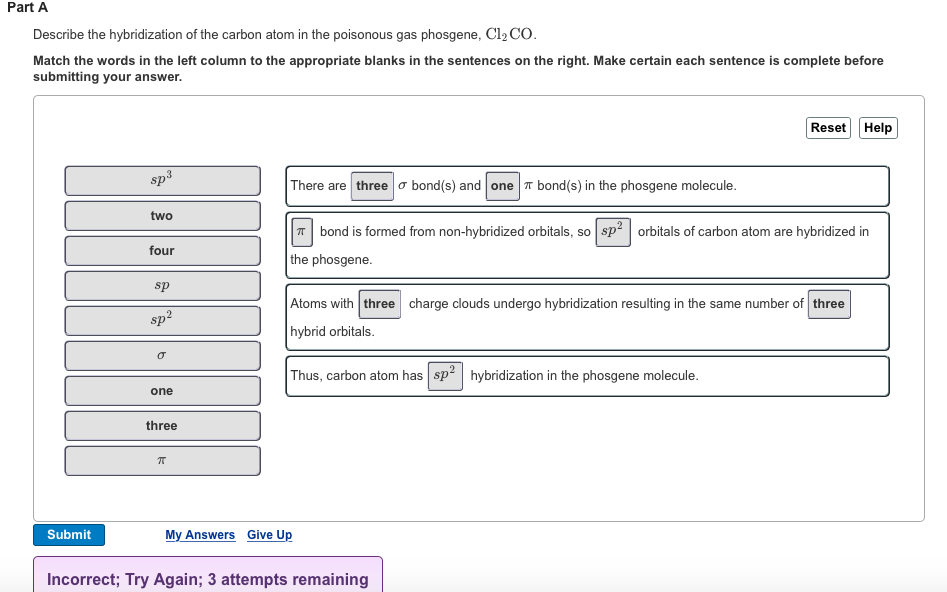
Part A Describe the hybridization of the carbon atom in the poisonous gas phosgene, Cl CO. Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer. Submit sp two four sp 2 sp one three There are three bond(s) and one bond(s) in the phosgene molecule. bond is formed from non-hybridized orbitals, so sp orbitals of carbon atom are hybridized the phosgene. Reset Help Atoms with three charge clouds undergo hybridization resulting in the same number of three hybrid orbitals. Thus, carbon atom has sp hybridization in the phosgene molecule. My Answers Give Up Incorrect; Try Again; 3 attempts remaining
Step by Step Solution
★★★★★
3.48 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
C1 0 FCI Therefore there are three sigma bonds 0 and one pi bond 7 in the ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


