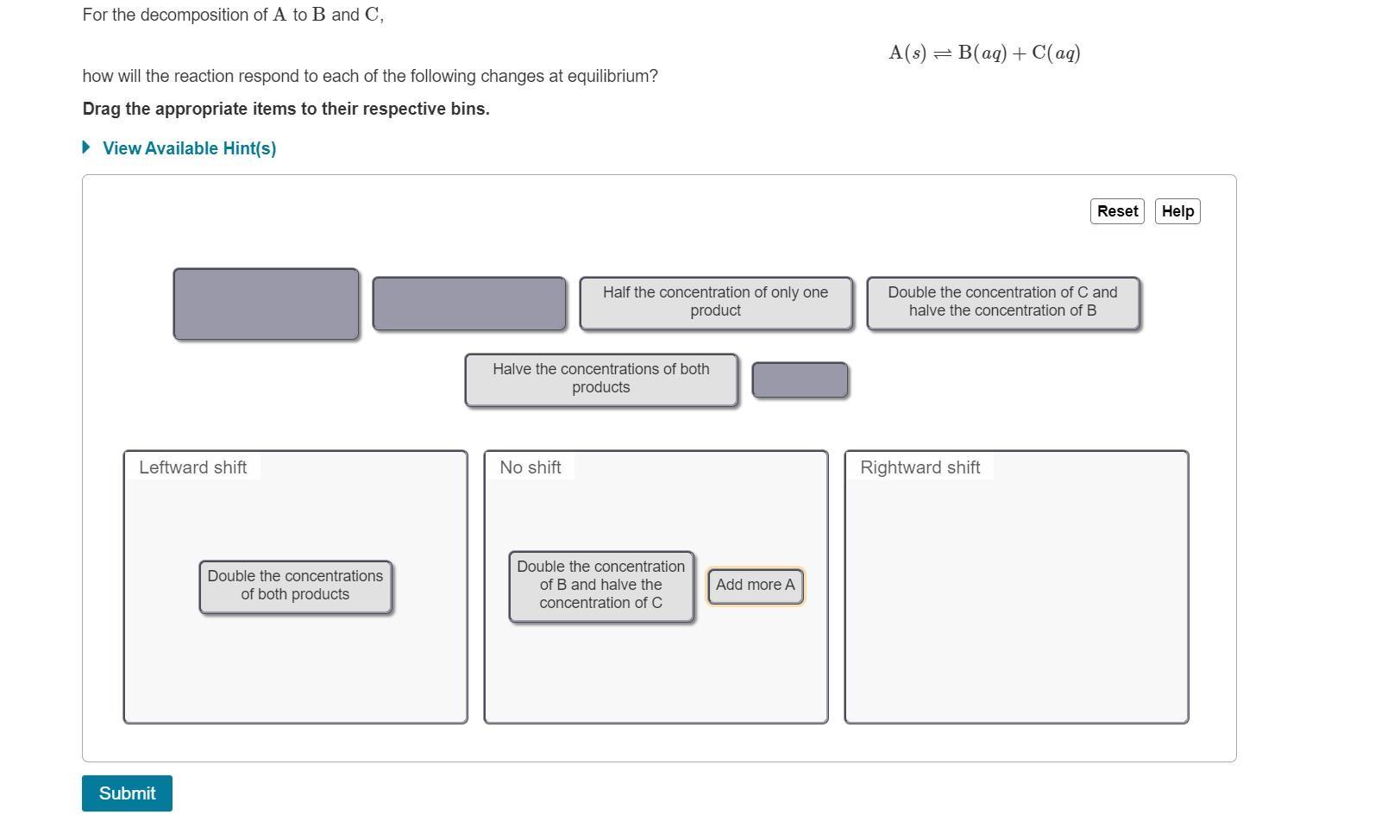
For the decomposition of A to B and C, A(s) = B(aq) + C(aq) how will the reaction respond to each of the following
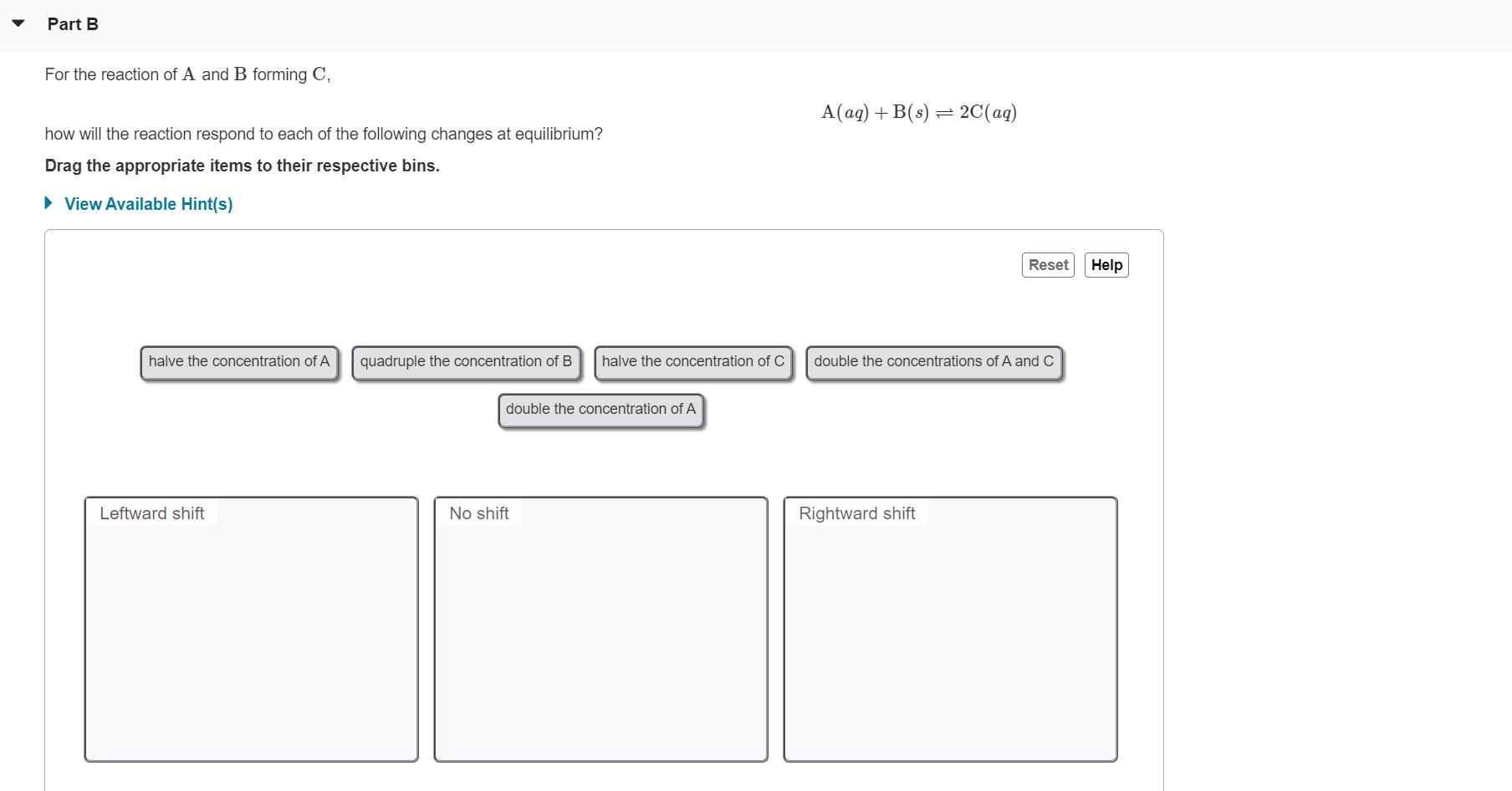
For the decomposition of A to B and C, A(s) = B(aq) + C(aq) how will the reaction respond to each of the following changes at equilibrium? Drag the appropriate items to their respective bins. View Available Hint(s) Reset Help Half the concentration of only one product Double the concentration of C and halve the concentration of B Halve the concentrations of both products Leftward shift No shift Rightward shift Double the concentration Double the concentrations of B and halve the Add more A of both products concentration of C Submit Part B For the reaction of A and B forming C, A(aq) + B(s) = 2C(ag) how will the reaction respond to each of the following changes at equilibrium? Drag the appropriate items to their respective bins. View Available Hint(s) Reset Help halve the concentration of A quadruple the concentration of B halve the concentration of C double the concentrations of A and C double the concentration of A Leftward shift No shift Rightward shift
Step by Step Solution
3.42 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started