Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part A Give the mass of the solute and the volume of the solvent for 1.70 L of 0.100 M (NH4)2SO4 solution, starting with
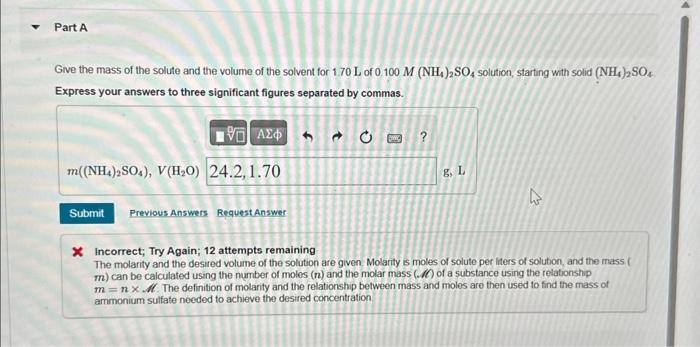
Part A Give the mass of the solute and the volume of the solvent for 1.70 L of 0.100 M (NH4)2SO4 solution, starting with solid (NH4)2SO4 Express your answers to three significant figures separated by commas. 195 m((NH,),SO4), V(H,O) 24.2,1.70 Submit Previous Answers Request Answer ? 8, L 4 * Incorrect; Try Again; 12 attempts remaining The molarity and the desired volume of the solution are given. Molarity is moles of solute per liters of solution, and the mass ( m) can be calculated using the number of moles (n) and the molar mass (.) of a substance using the relationship m = nxM. The definition of molarity and the relationship between mass and moles are then used to find the mass of ammonium sulfate needed to achieve the desired concentration
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To solve this problem we need to calculate both the mass of the solute NH42SO4 ammonium sulfate and ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


