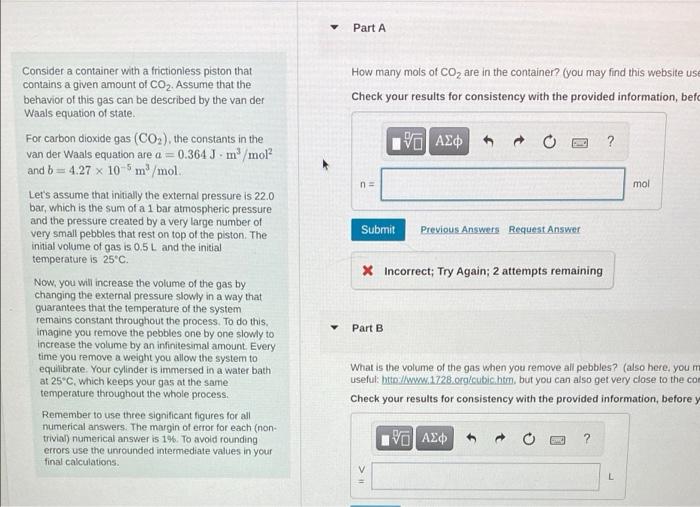
Part A How many mols of CO2 are in the container? (you may find this website use Check your results for consistency with the provided information, bet | ? n = mol Submit Previous Answers Request Answer Consider a container with a frictionless piston that contains a given amount of CO2. Assume that the behavior of this gas can be described by the van der Waals equation of state For carbon dioxide gas (CO2), the constants in the van der Waals equation are a = 0.364 J. m/mol? and b = 4.27 x 10-5m/mol Let's assume that initially the external pressure is 22.0 bar, which is the sum of a 1 bar atmospheric pressure and the pressure created by a very large number of very small pebbles that rest on top of the piston. The initial volume of gas is 0.5L and the initial temperature is 25C Now, you will increase the volume of the gas by changing the external pressure slowly in a way that guarantees that the temperature of the system remains constant throughout the process. To do this, imagine you remove the pebbles one by one slowly to increase the volume by an infinitesimal amount Every time you remove a weight you allow the system to equilibrate. Your cylinder is immersed in a water bath at 25C, which keeps your gas at the same temperature throughout the whole process. Remember to use three significant figures for all numerical answers. The margin of erfor foreach (non trivial) numerical answer is 1%. To avoid rounding errors use the unrounded intermediate values in your X Incorrect; Try Again: 2 attempts remaining Part B What is the volume of the gas when you remove all pebbles? (also here, you m useful: htto://www.1728.org/cubic.htm, but you can also get very close to the cor Check your results for consistency with the provided information, before y ? final calculations. V L Part A How many mols of CO2 are in the container? (you may find this website use Check your results for consistency with the provided information, bet | ? n = mol Submit Previous Answers Request Answer Consider a container with a frictionless piston that contains a given amount of CO2. Assume that the behavior of this gas can be described by the van der Waals equation of state For carbon dioxide gas (CO2), the constants in the van der Waals equation are a = 0.364 J. m/mol? and b = 4.27 x 10-5m/mol Let's assume that initially the external pressure is 22.0 bar, which is the sum of a 1 bar atmospheric pressure and the pressure created by a very large number of very small pebbles that rest on top of the piston. The initial volume of gas is 0.5L and the initial temperature is 25C Now, you will increase the volume of the gas by changing the external pressure slowly in a way that guarantees that the temperature of the system remains constant throughout the process. To do this, imagine you remove the pebbles one by one slowly to increase the volume by an infinitesimal amount Every time you remove a weight you allow the system to equilibrate. Your cylinder is immersed in a water bath at 25C, which keeps your gas at the same temperature throughout the whole process. Remember to use three significant figures for all numerical answers. The margin of erfor foreach (non trivial) numerical answer is 1%. To avoid rounding errors use the unrounded intermediate values in your X Incorrect; Try Again: 2 attempts remaining Part B What is the volume of the gas when you remove all pebbles? (also here, you m useful: htto://www.1728.org/cubic.htm, but you can also get very close to the cor Check your results for consistency with the provided information, before y ? final calculations. V L