Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part A Identify which of the following equations are balanced. Check all that apply. 2KMnO4(s)K MnO4(s) + MnO2 (s) + O2(g) 2KClO3(s)2KCl(s) + O2(g)
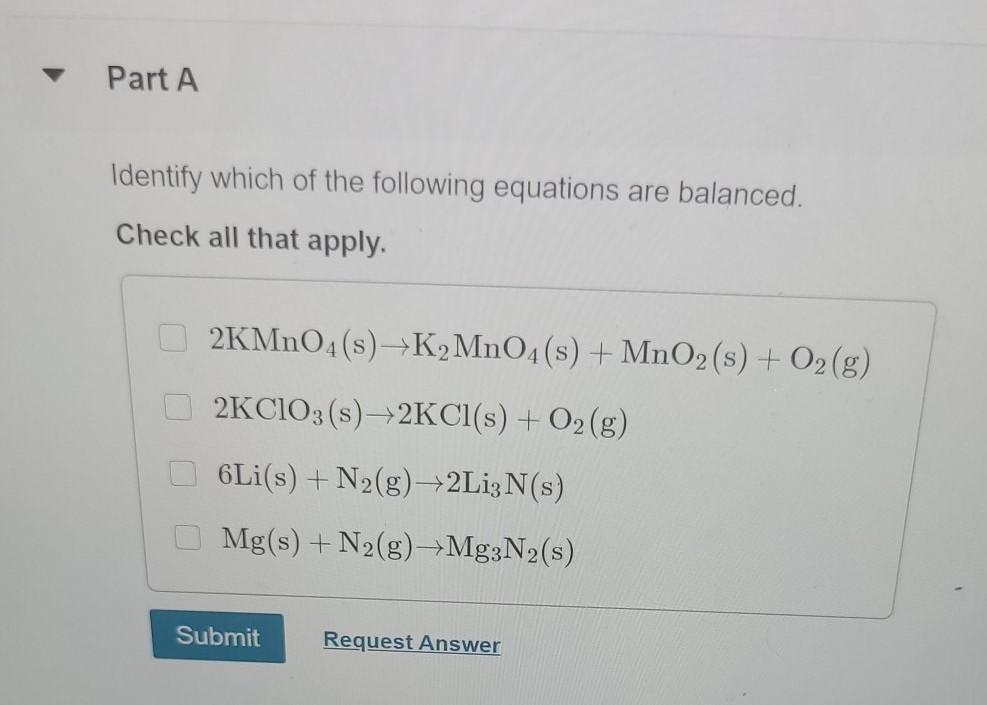

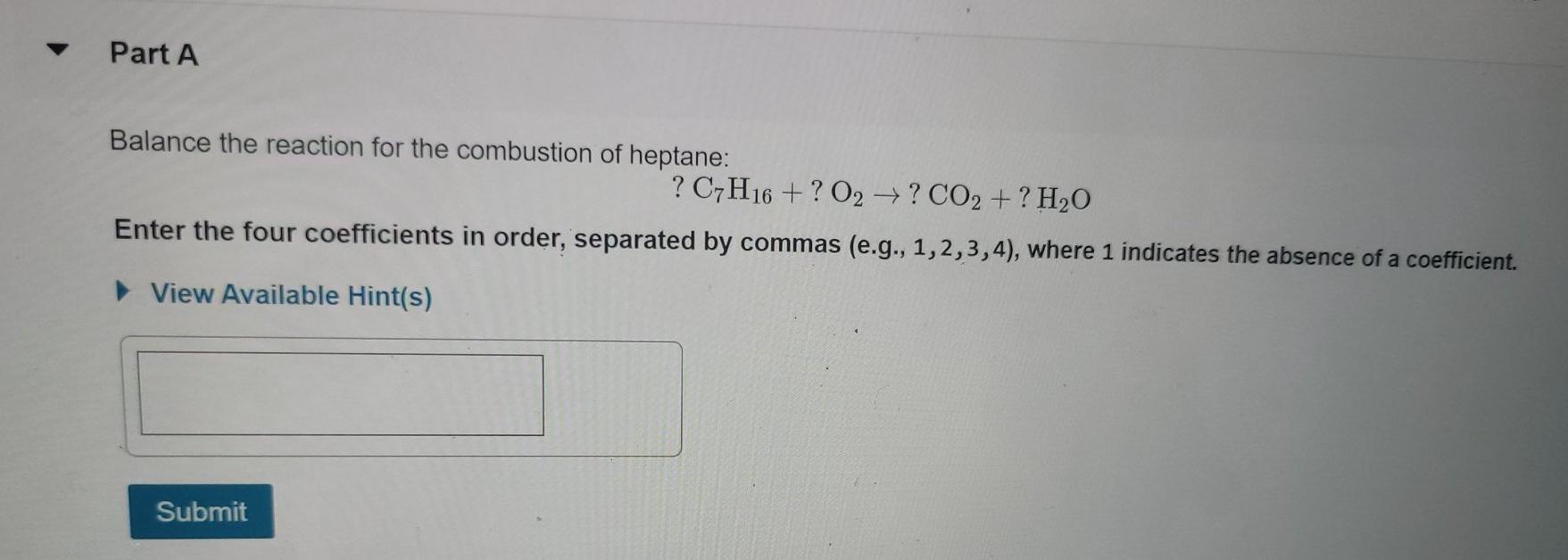
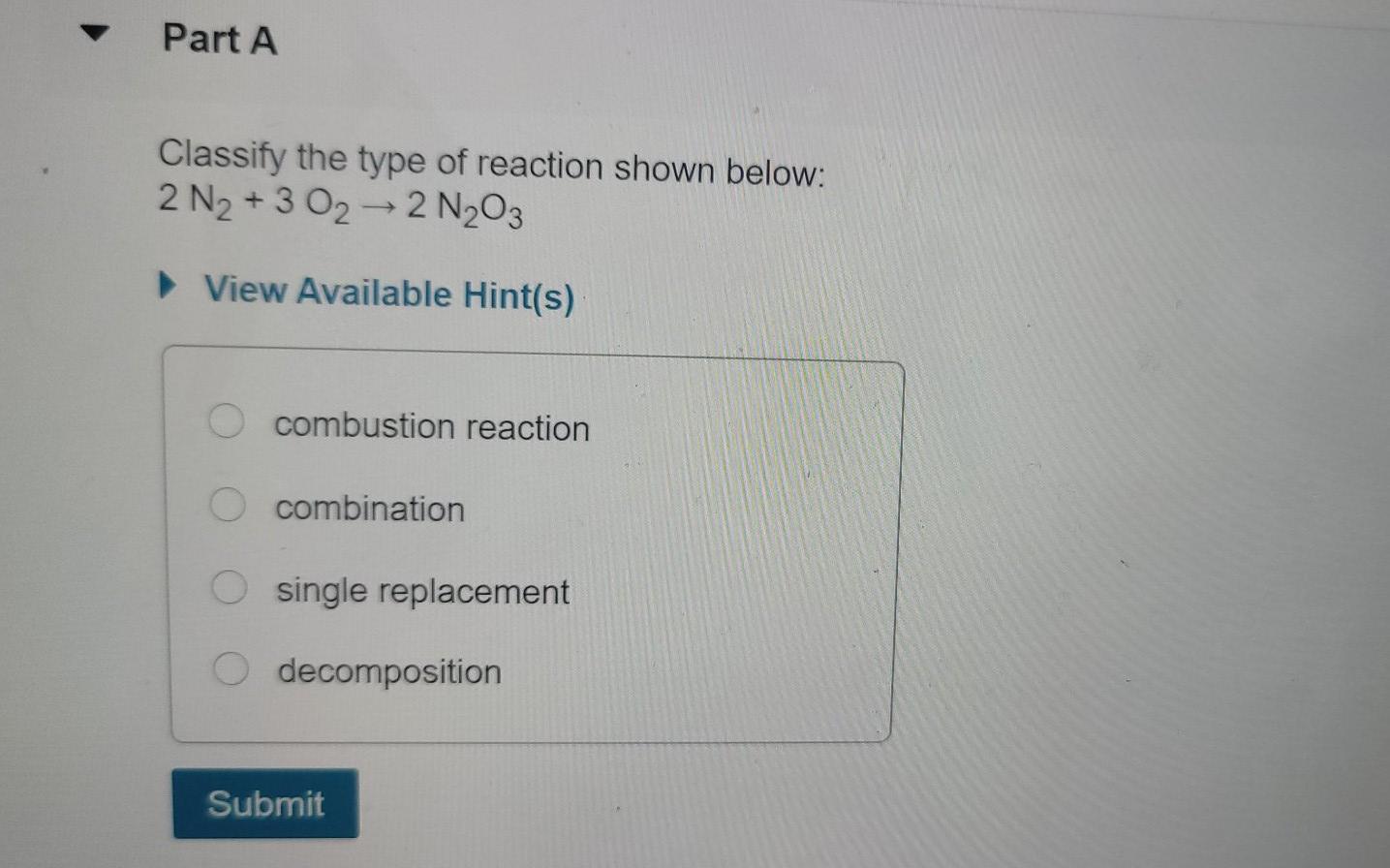

Part A Identify which of the following equations are balanced. Check all that apply. 2KMnO4(s)K MnO4(s) + MnO2 (s) + O2(g) 2KClO3(s)2KCl(s) + O2(g) 6Li(s) + N(g) 2Li; N(s) Mg(s) + N(g) Mg3N2(s) Submit Request Answer Part A Notice that "SO4" appears in two different places in this chemical equation. SO2 is a polyatomic ion called sulfate. What number should be placed in front of CaSO4 to give the same total number of sulfate ions on each side of the equation? ?CaSO4 + AlCl3 CaCl2 + Al2(SO4)3 Express your answer numerically as an integer. View Available Hint(s) ? = CaCl2 + Al2(SO4)3 Submit Part A Balance the reaction for the combustion of heptane: ? C7H16 +? O2 ? CO2 +? HO Enter the four coefficients in order, separated by commas (e.g., 1,2,3,4), where 1 indicates the absence of a coefficient. View Available Hint(s) Submit Part A Classify the type of reaction shown below: 2 N +3 022 NO3 View Available Hint(s) combustion reaction O combination single replacement decomposition Submit Part A Classify the following reactions as synthesis, decomposition, single-displacement, or double-displacement reactions. Drag the items to their respective bins. View Available Hint(s) C3H6 + Br2 C3H6Br2 Synthesis (combination) Na2CO3 + 2HCI 2NaCl + H2CO3 CaCO3 CaCO + CO2 Zn + HSO4 ZnSO4 + H Decomposition 2HC1 H + Cl Hg2 (NO3)2 + 2NaCl 2NaNO3 + Hg2 Cl Ni+ Cl-NiCl2 Single-displacement Reset 0 +0 Double-displacement Help
Step by Step Solution
★★★★★
3.56 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for the above question is provided be...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


