Answered step by step
Verified Expert Solution
Question
1 Approved Answer
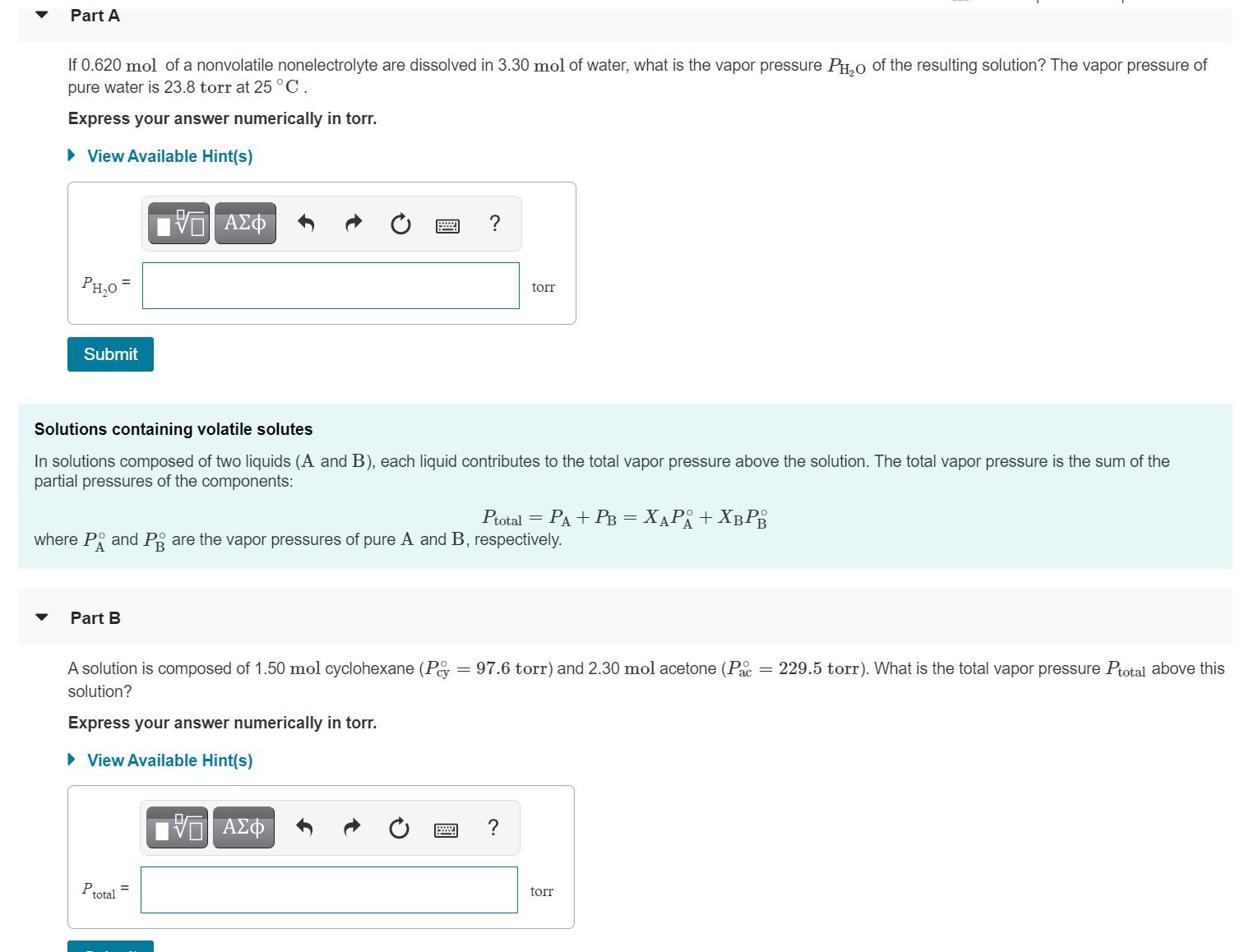
Part A If 0.620 mol of a nonvolatile nonelectrolyte are dissolved in 3.30 mol of water, what is the vapor pressure PH,0 of the
Part A If 0.620 mol of a nonvolatile nonelectrolyte are dissolved in 3.30 mol of water, what is the vapor pressure PH,0 of the resulting solution? The vapor pressure of pure water is 23.8 torr at 25 C. Express your answer numerically in torr. View Available Hint(s) ? PH,0 = torr Submit Solutions containing volatile solutes In solutions composed of two liquids (A and B), each liquid contributes to the total vapor pressure above the solution. The total vapor pressure is the sum of the partial pressures of the components: Potal 3D P + 3 + = where P and Pg are the vapor pressures of pure A and B, respectively. Part B A solution is composed of 1.50 mol cyclohexane (Pey 97.6 torr) and 2.30 mol acetone (P = 229.5 torr). What is the total vapor pressure Ptotal above this solution? Express your answer numerically in torr. View Available Hint(s) V AZO ? Ptotal torr
Step by Step Solution
★★★★★
3.40 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started