Answered step by step
Verified Expert Solution
Question
1 Approved Answer
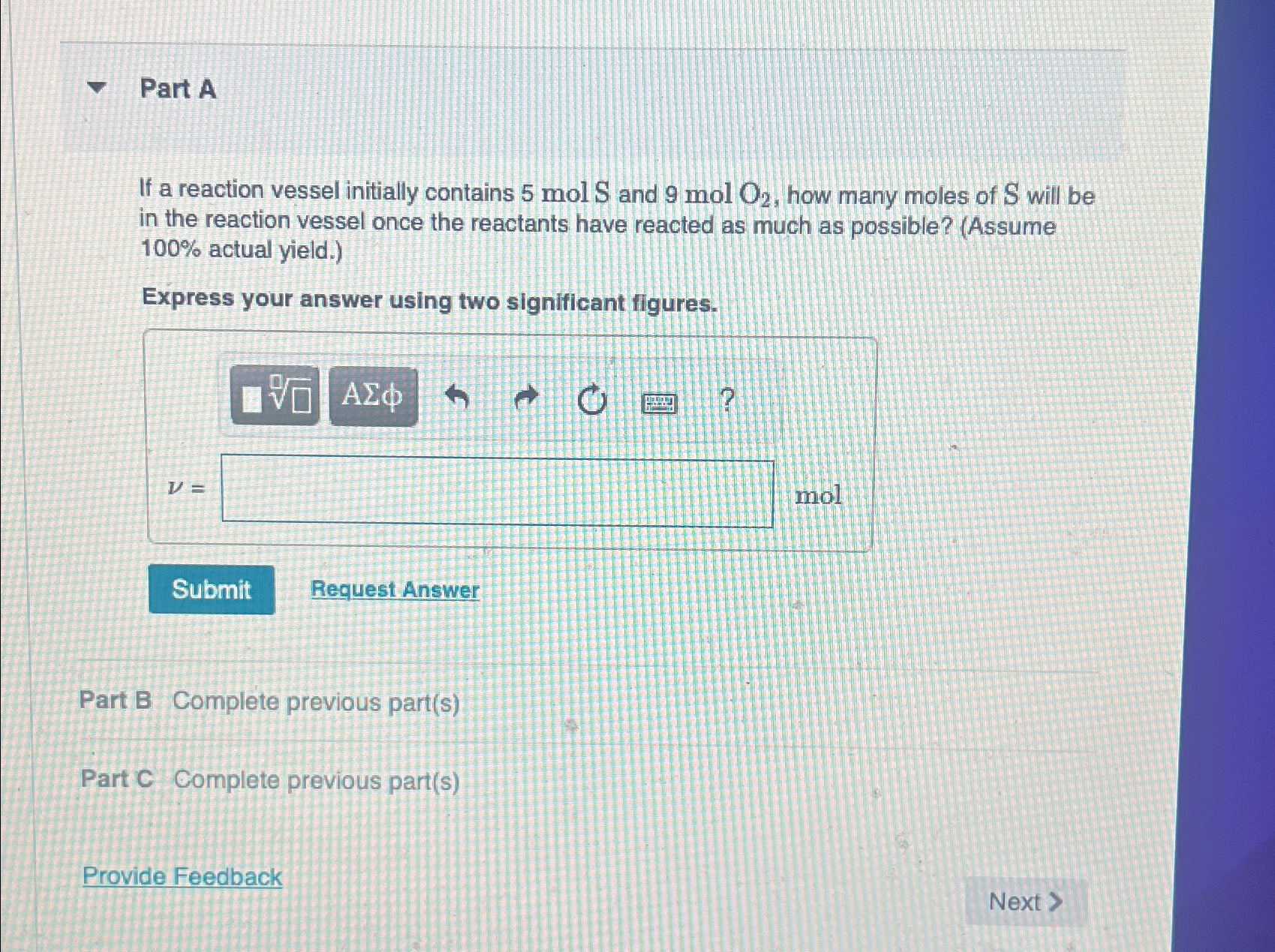
Part A If a reaction vessel initially contains 5molS and 9molO_(2) , how many moles of S will be in the reaction vessel once the
Part A\ If a reaction vessel initially contains
5molSand
9molO_(2), how many moles of
Swill be in the reaction vessel once the reactants have reacted as much as possible? (Assume
100%actual yield.)\ Express your answer using two significant figures.\
\ u = nol \ Request Answer\ Part B Complete previous part(s)\ Part C Complete previous part(s)\ Provide Feedback
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


