Answered step by step
Verified Expert Solution
Question
1 Approved Answer
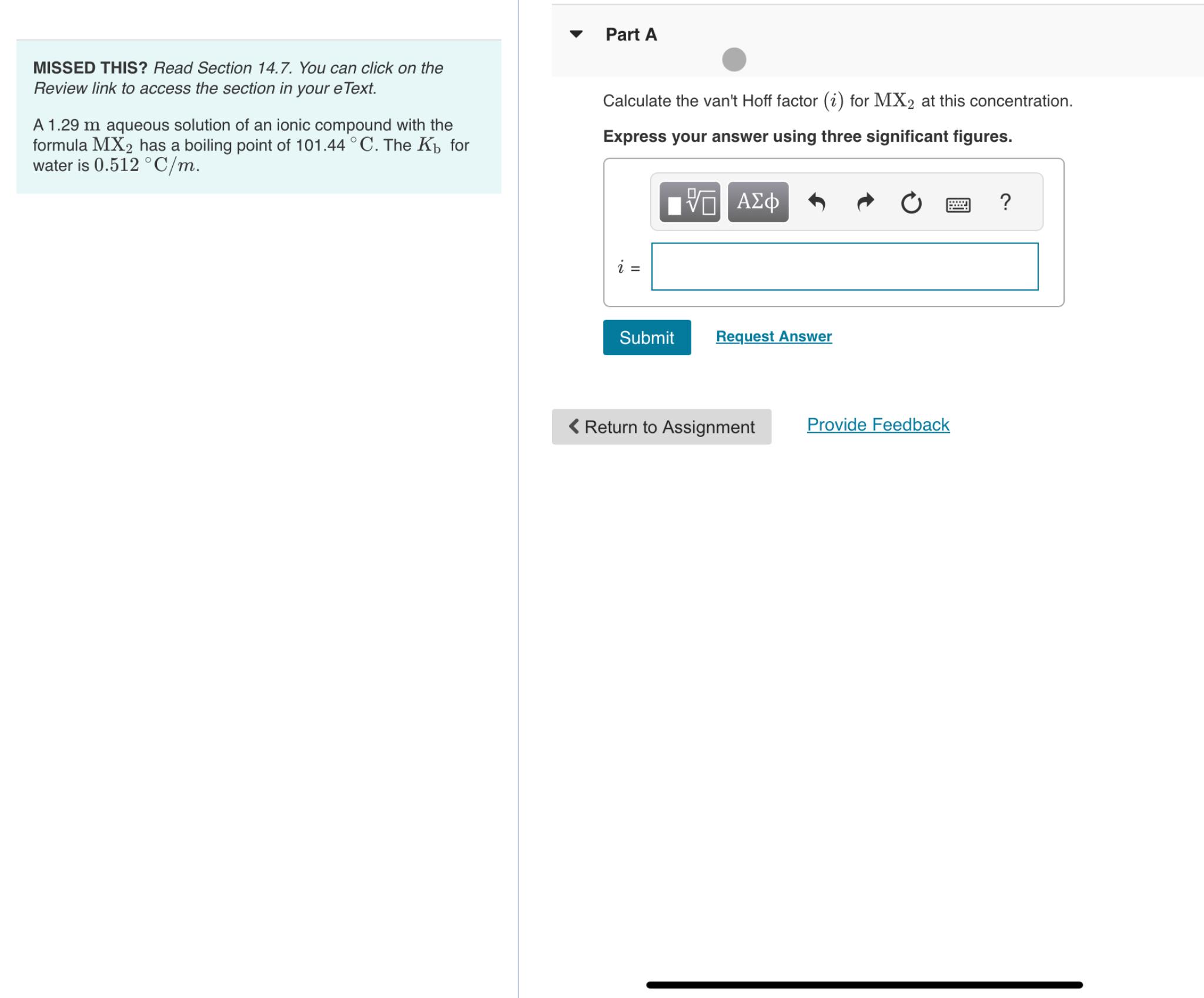
Part A MISSED THIS? Read Section 14.7. You can click on the Review link to access the section in your eText. A 1.29m aqueous solution
Part A\ MISSED THIS? Read Section 14.7. You can click on the Review link to access the section in your eText.\ A
1.29maqueous solution of an ionic compound with the formula
Mx_(2)has a boiling point of
101.44\\\\deg C. The
K_(b)for water is
0.512\\\\deg (C)/(m).\ Calculate the van't Hoff factor
(i)for
Mx_(2)at this concentration.\ Express your answer using three significant figures.\
i=\ Provide Feedback
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


