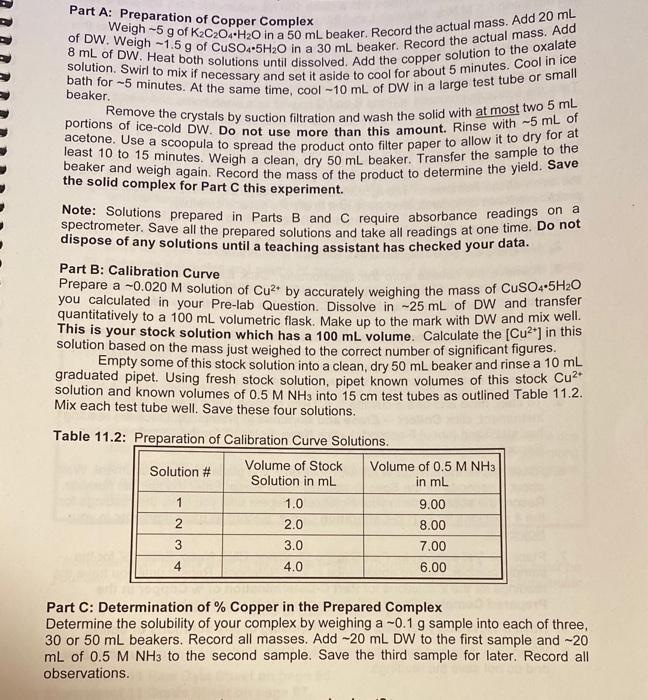
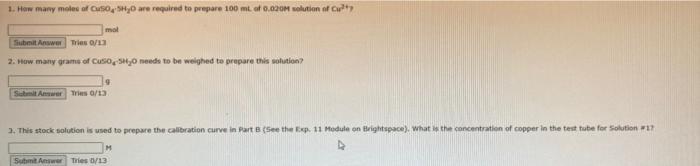
Part A: Preparation of Copper Complex beaker. of DW. Weigh -1.5 g of CuSO4.5H2O in a 30 mL beaker. Record the actual mass. Add Weigh 5 g of K2C2O4+H2O in a 50 mL beaker. Record the actual mass. Add 20 mL 8 mL of DW. Heat both solutions until dissolved. Add the copper solution to the oxalate bath for -5 minutes. At the same time, cool-10 mL of DW in a large test tube or small solution. Swirl to mix if necessary and set it aside to cool for about 5 minutes. Cool in ice portions of ice-cold DW. Do not use more than this amount. Rinse with -5 mL of Remove the crystals by suction filtration and wash the solid with at most two 5 mL acetone. Use a scoopula to spread the product onto filter paper to allow it to dry for at beaker and weigh again. Record the mass of the product to determine the yield. Save the solid complex for Part C this experiment. Note: Solutions prepared in Parts B and C require absorbance readings on a spectrometer. Save all the prepared solutions and take all readings at one time. Do not dispose of any solutions until a teaching assistant has checked your data. Part B: Calibration Curve Prepare a -0.020 M solution of Cut by accurately weighing the mass of CuSO4-5H20 you calculated in your Pre-lab Question. Dissolve in -25 mL of DW and transfer quantitatively to a 100 mL volumetric flask. Make up to the mark with DW and mix well. This is your stock solution which has a 100 mL volume. Calculate the [Cu?*) in this solution based on the mass just weighed to the correct number of significant figures. Empty some of this stock solution into a clean, dry 50 mL beaker and rinse a 10 mL graduated pipet. Using fresh stock solution, pipet known volumes of this stock Cu2* solution and known volumes of 0.5 M NH3 into 15 cm test tubes as outlined Table 11.2. Mix each test tube well. Save these four solutions. Table 11.2: Preparation of Calibration Curve Solutions. Solution # Volume of Stock Volume of 0.5 M NH3 Solution in mL in mL 1 1.0 9.00 2 8.00 3 3.0 7.00 4 4.0 6.00 2.0 Part C: Determination of % Copper in the Prepared Complex Determine the solubility of your complex by weighing a -0.1 g sample into each of three, 30 or 50 ml beakers. Record all masses. Add -20 mL DW to the first sample and -20 mL of 0.5 M NH3 to the second sample. Save the third sample for later. Record all observations. 1. How many moles af 150, 5740 are required to prepare 100 mL at 0,020M solution of Cu? mal Submit Answer Tries/13 2. How many grams of Cuso needs to be weighed to prepare this solution? Schmit Answers 0/13 3. This stock solution is used to prepare the calibration curve in Part (See the Ep 11 Module on Brightspace). What is the concentration of copper in the test tube for Solution 1 M Submit Answer Tries 0/13