Question
Part A Select the sketches of a 3d orbital. Check all that apply 0 0 0 *% k Submit Previous Answers Request Answer X
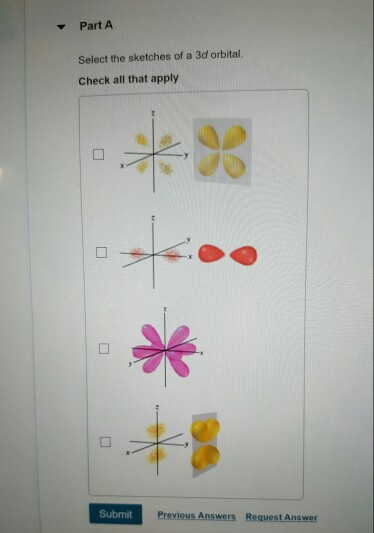
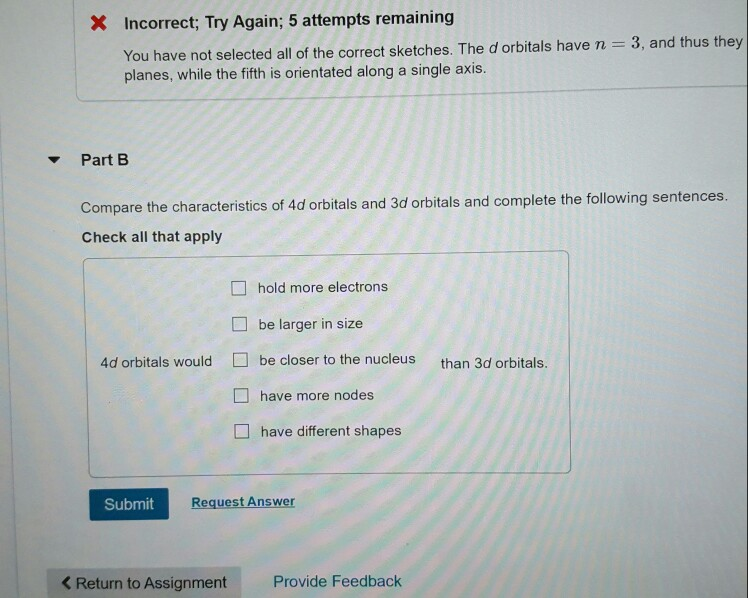
Part A Select the sketches of a 3d orbital. Check all that apply 0 0 0 *% k Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining You have not selected all of the correct sketches. The d orbitals have n = 3, and thus they planes, while the fifth is orientated along a single axis. Part B Compare the characteristics of 4d orbitals and 3d orbitals and complete the following sentences. Check all that apply 4d orbitals would hold more electrons be larger in size be closer to the nucleus than 3d orbitals. have more nodes < Return to Assignment have different shapes Submit Request Answer Provide Feedback
Step by Step Solution
3.45 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Basic Marketing Research
Authors: Tom J. Brown, Tracy A. Suter, Gilbert A. Churchill
8th edition
1133188540, 978-1111525293, 1111525293, 978-1305178571, 1305178572, 978-1133188544
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



