Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part A) Use bond enthalpies from the table below to determine whether CH4(g), CH3OH(g), H2CO(g), or HCOOH(g) produces the most energy per gram when burned
Part A) Use bond enthalpies from the table below to determine whether CH4(g), CH3OH(g), H2CO(g), or HCOOH(g) produces the most energy per gram when burned completely in O2(g) to give CO2(g) and H2O(g).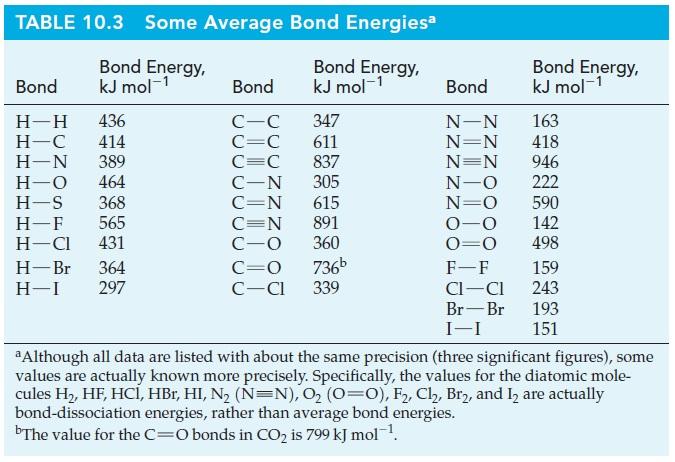
a. CH4(g)
b. CH3OH(g)
c. HCOOH(g)
d. H2CO(g)
e. none of these
Part B) Describe the relationship between the oxidation state of carbon and the heat of combustion (in kJkg1kJkg1 or kJmol1kJmol1)?
The more the oxidation state of the carbon, the energy that is generated per gram of that material.
options: negative, positive, less, more
TABLE 10.3 Some Average Bond Energiesa Bond Energy, Bond Energy, Bond Energy Bond kJ mol-1 Bond kJ mol-1 Bond kJ mol-1 H-H 436 C-C 347 N-N 163 H-C 414 C=C 611 N=N 418 H-N 389 C=C 837 N=N 946 H-O 464 C-N 305 N-O 222 H-S 368 CEN 615 N=0 590 H-F 565 CON 891 0-0 142 H-CI 431 C-0 360 O=O 498 H- Br 364 CEO 736b F-F 159 H-1 297 C-Cl 339 C1-C1 243 Br-Br 193 I-I 151 a Although all data are listed with about the same precision (three significant figures), some values are actually known more precisely. Specifically, the values for the diatomic mole- cules H2, HF, HC, HBr, HI, N2 (N=N), 02 (O=O), F2, Cl, Bry, and I are actually bond-dissociation energies, rather than average bond energies. bThe value for the C=O bonds in CO2 is 799 kJ mollStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


