Answered step by step
Verified Expert Solution
Question
1 Approved Answer
part b and c please Feed Mixer 2 Reactor 3 1 Separator Product 4 I 5 The feed stream (stream 1), containing pure butane (CH),
part b and c please 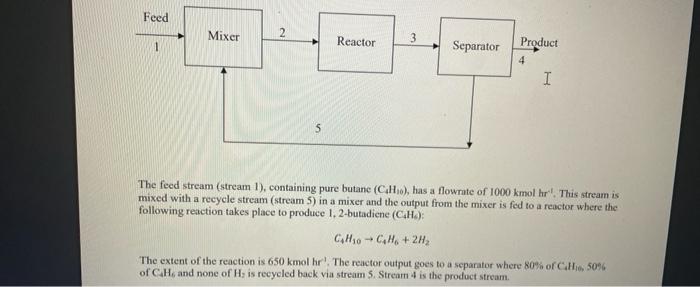

Feed Mixer 2 Reactor 3 1 Separator Product 4 I 5 The feed stream (stream 1), containing pure butane (CH), has a flowrate of 1000 kmol hrl. This stream is mixed with a recycle stream (stream 5) in a mixer and the output from the mixer is fed to a reactor where the following reaction takes place to produce 1.2-butadiene (CH): Calo -CM+2H The extent of the reaction is 650 kmol hrThe reactor output goes to a separator where 80% ofC.H. 50% of CH, and none of His recycled back via stream 5 Stream 4 is the product stream The feed stream (stream 1), containing pure butane (C4H16), has a flowrate of 1000 kmol hrl. This stream is mixed with a recycle stream (stream 5) in a mixer and the output from the mixer is fed to a reactor where the following reaction takes place to produce 1, 2-butadiene (C4H): - CHOCH6 + 2H2 The extent of the reaction is 650 kmol hr'. The reactor output goes to a separator where 80% of CH1, 50% of Cal and none of H2 is recycled back via stream 5. Stream 4 is the product stream. PART A: Mass Balance: Write the mass balance for this problem and submit it as a part of the report. Carry out degrees of freedom analysis and if it is not zero then make it zero by specifying some variables or removing some specification. Submit degrees of freedom analysis in the report. PART B: Equation Oriented Process Flowsheeting - using GAMS as a modelling tool Formulate this problem in GAMS and solve it using the CNS solver. Analyse and discuss the effect of the initial guess. Submit the gms and .Ist files. PART C: Sequential Modular Process Flowsheeting - using GAMS as a programming tool Page 1 of 2 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


