Answered step by step
Verified Expert Solution
Question
1 Approved Answer
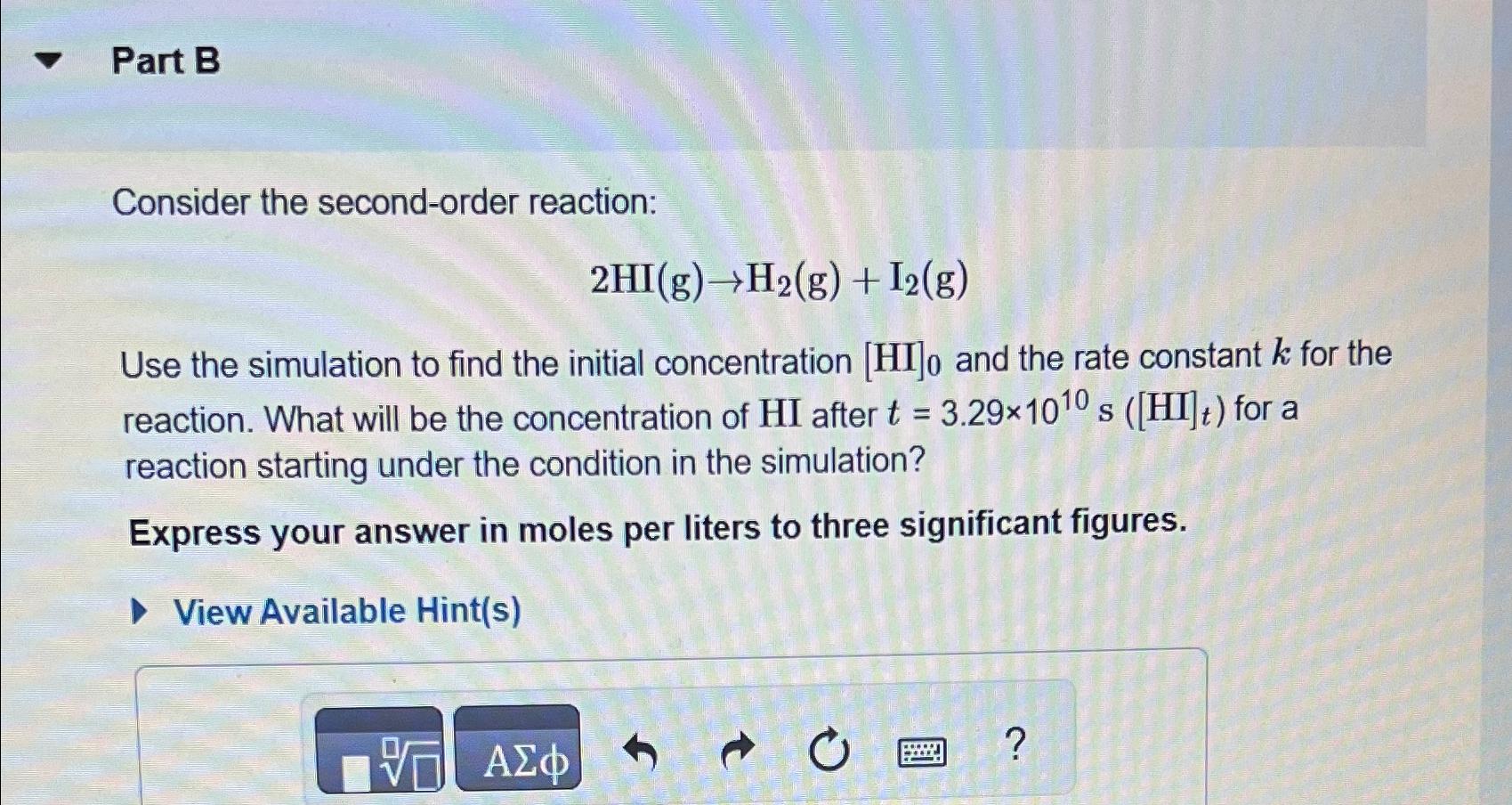
Part B Consider the second-order reaction: 2HI(g)->H_(2)(g)+I_(2)(g) Use the simulation to find the initial concentration [HI]_(0) and the rate constant k for the reaction.
Part B\ Consider the second-order reaction:\
2HI(g)->H_(2)(g)+I_(2)(g)\ Use the simulation to find the initial concentration
[HI]_(0)and the rate constant
kfor the reaction. What will be the concentration of
HIafter
t=3.29\\\\times 10^(10)s([HI]_(t))for a reaction starting under the condition in the simulation?\ Express your answer in moles per liters to three significant figures.\ View Available Hint(s)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


