Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part B rate of reaction= 1.5x10^-3 molL^-1s^-1 - Part C The half-life of a reaction, 11/2, is the time required for one-half of a reactant
Part B rate of reaction= 1.5x10^-3 molL^-1s^-1 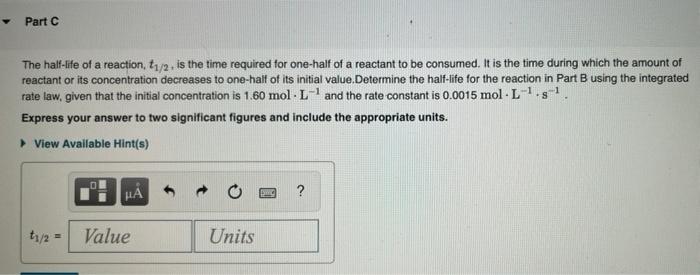
- Part C The half-life of a reaction, 11/2, is the time required for one-half of a reactant to be consumed. It is the time during which the amount of reactant or its concentration decreases to one-half of its initial value.Determine the half-life for the reaction in Part B using the integrated rate law, given that the initial concentration is 1.60 mol. L-and the rate constant is 0.0015 mol. L-1.g! Express your answer to two significant figures and include the appropriate units. View Available Hint(s) ? th/3 = Value Units 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


