Answered step by step
Verified Expert Solution
Question
1 Approved Answer
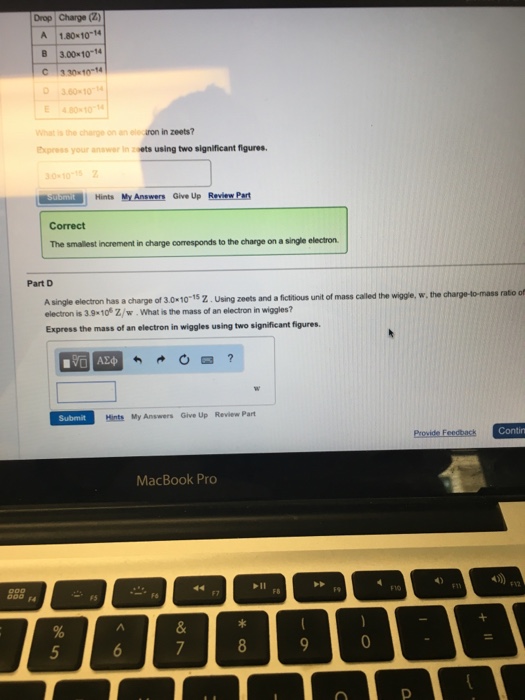
Part D Drop Charge (2) A 1.8010-14 B 3.00-10-14 C330-10-14 D3.60-10-14 4.80-10-14 E What is the charge on an electron in zeets? Express your answer
 Part D
Part D Drop Charge (2) A 1.8010-14 B 3.00-10-14 C330-10-14 D3.60-10-14 4.80-10-14 E What is the charge on an electron in zeets? Express your answer in zoets using two significant figures. 000 000 14 30-10-15 2 submit Correct The smallest increment in charge corresponds to the charge on a single electron. Part D A single electron has a charge of 3.0x10-15 Z. Using zeets and a fictitious unit of mass called the wiggle, w, the charge-to-mass ratio off electron is 3.9x10 Z/w. What is the mass of an electron in wiggles? Express the mass of an electron in wiggles using two significant figures. Hints My Answers Give Up Review Part % 5 195) Submit Hints My Answers Give Up Review Part F5 A 6 MacBook Pro & 7 ? * 8 || FB 9 F9 C 0 F10 Provide Feedback Contin P 4) FI +11 F12
Step by Step Solution
★★★★★
3.41 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
Charge of Single electron 6 charge to mass ratio 39 x ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


