Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part D What is the reaction rate at 1 0 0 0 K if N O is decreased to 0 . 0 1 4 M
Part D
What is the reaction rate at if is decreased to and is increased to
Express the rate in moles per liter per second to two significant figures.
Previous Answers
Request Answer
Incorrect; Try Again
The given concentrations along with the provided rate constant can be substituted into the rate law determined in Part A to calculate the overall reaction rate.
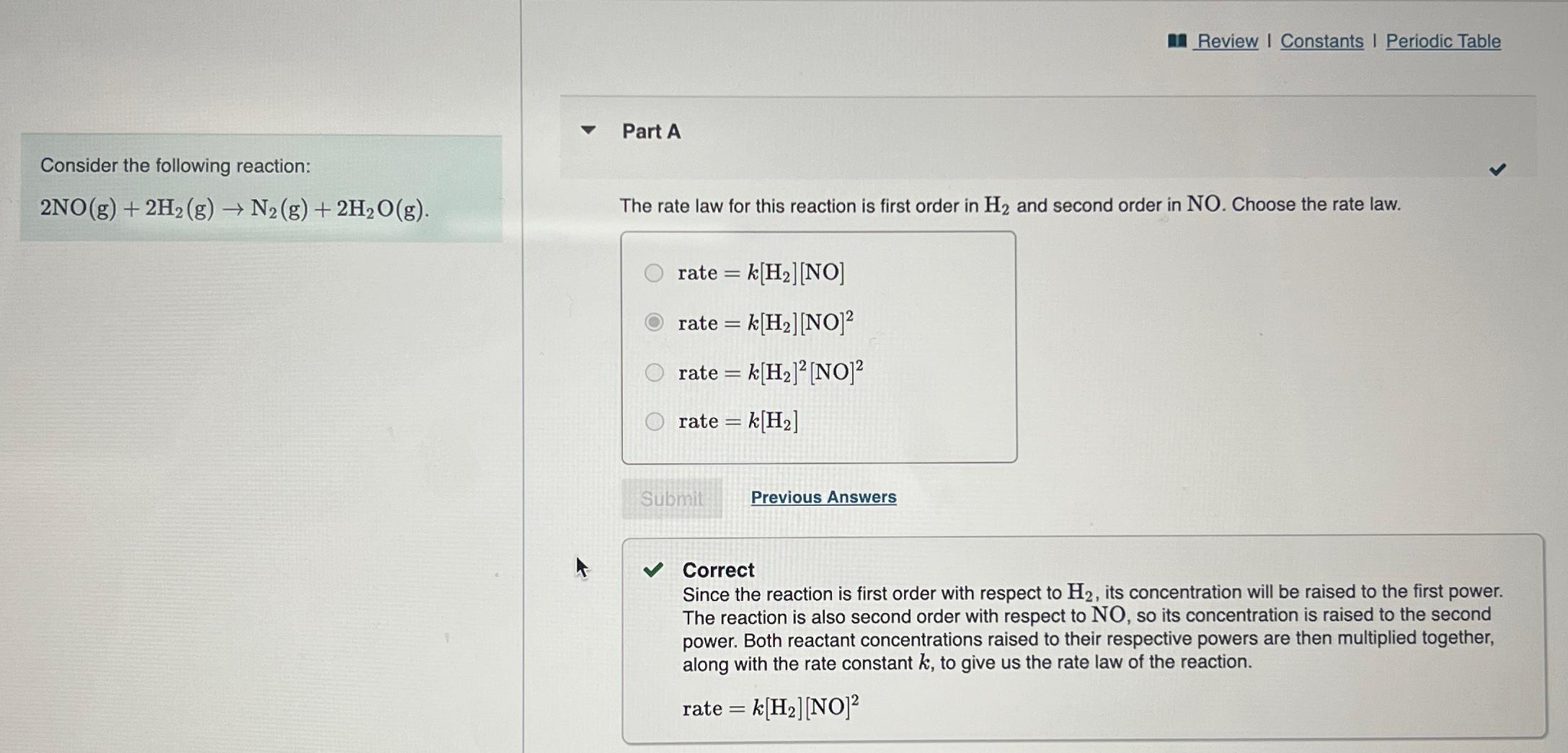
Review I Constants I Periodic Table
Part A
Consider the following reaction:
The rate law for this reaction is first order in and second order in Choose the rate law.
rate
rate
rate
rate
Previous Answers
Correct
Since the reaction is first order with respect to its concentration will be raised to the first power. The reaction is also second order with respect to NO so its concentration is raised to the second power. Both reactant concentrations raised to their respective powers are then multiplied together, along with the rate constant to give us the rate law of the
reaction.
rate
PLEASE SOLVE PART D IN THE DESCRIPTION
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


