Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part I: Mass and Volume Measurements for Water Table 1: Mass, Volume, and Density of Water Volume of water (ml) Mass (g) 27.9 27.281
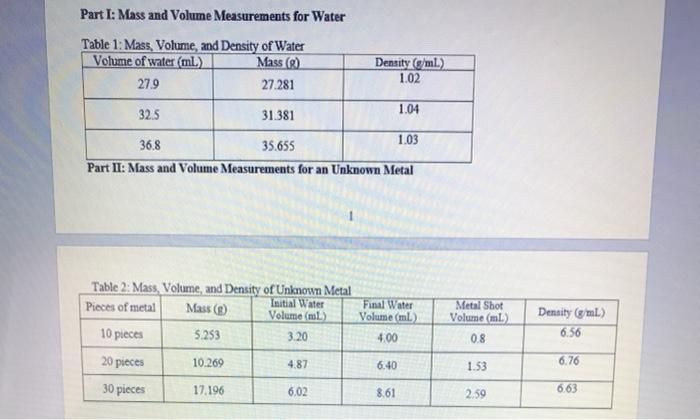
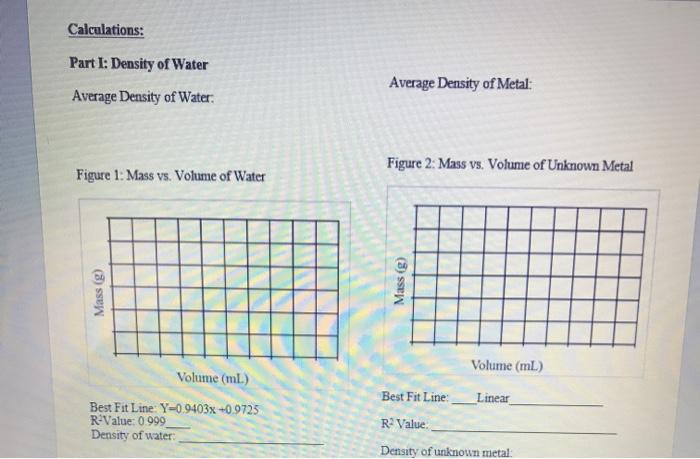
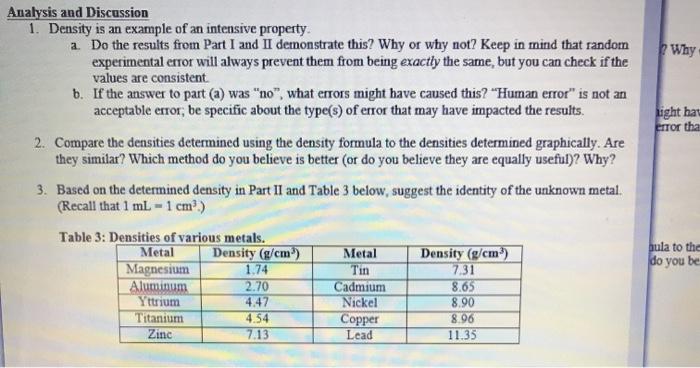
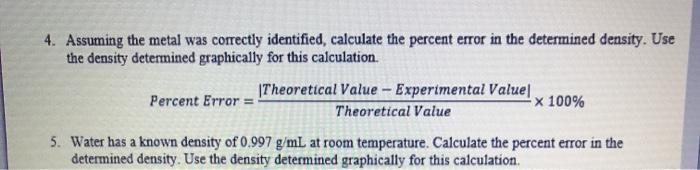
Part I: Mass and Volume Measurements for Water Table 1: Mass, Volume, and Density of Water Volume of water (ml) Mass (g) 27.9 27.281 32.5 31.381 36.8 35.655 Part II: Mass and Volume Measurements for an Unknown Metal Table 2: Mass, Volume, and Density of Unknown Metal Initial Water Pieces of metal Mass (g) Volume (ml) 10 pieces 5.253 3.20 20 pieces 30 pieces 10.269 17.196 4.87 6.02 Density (g/ml.) 1.02 6.40 1.04 Final Water Volume (ml) 4.00 8.61 1.03 Metal Shot Volume (ml) 0.8 1.53 2.59 Density (g/mL) 6.56 6.76 6.63 Calculations: Part I: Density of Water Average Density of Water: Figure 1: Mass vs. Volume of Water Mass (g) Volume (ml.) Best Fit Line: Y-0.9403x+0.9725 R-Value: 0.999 Density of water: Average Density of Metal: Figure 2: Mass vs. Volume of Unknown Metal Mass (g) Volume (ml) Best Fit Line: Linear R Value Density of unknown metal: Analysis and Discussion 1. Density is an example of an intensive property. a. Do the results from Part I and II demonstrate this? Why or why not? Keep in mind that random experimental error will always prevent them from being exactly the same, but you can check if the values are consistent. b. If the answer to part (a) was "no", what errors might have caused this? "Human error" is not an acceptable error; be specific about the type(s) of error that may have impacted the results. 2. Compare the densities determined using the density formula to the densities determined graphically. Are they similar? Which method do you believe is better (or do you believe they are equally useful)? Why? 3. Based on the determined density in Part II and Table 3 below, suggest the identity of the unknown metal. (Recall that 1 mL-1 cm.) Table 3: Densities of various metals. Metal Magnesium Aluminum Yttrium Titanium Zinc Density (g/cm) 1.74 2.70 4.47 4.54 7.13 Metal Tin Cadmium Nickel Copper Lead Density (g/cm) 7.31 8.65 8.90 8.96 11.35 Why ight hav error tha bula to the do you be 4. Assuming the metal was correctly identified, calculate the percent error in the determined density. Use the density determined graphically for this calculation. Percent Error: Theoretical Value - Experimental Value Theoretical Value -x 100% 5. Water has a known density of 0.997 g/mL at room temperature. Calculate the percent error in the determined density. Use the density determined graphically for this calculation.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Density Mass Volume Mass Density x Volume This equation is in the form of y mx Therefore if we plot ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


