Answered step by step
Verified Expert Solution
Question
1 Approved Answer
part ii and b please Antoine coefficients ( P in mmHg,T in K, log to base e ): Methanol: A=18.588,B=3626.6,C=34.29 Water: A=18.304,B=3816.4,C=46.13 Ethanol: A=18.9119,B=3803.98,C=41.68 Margules
part ii and b please 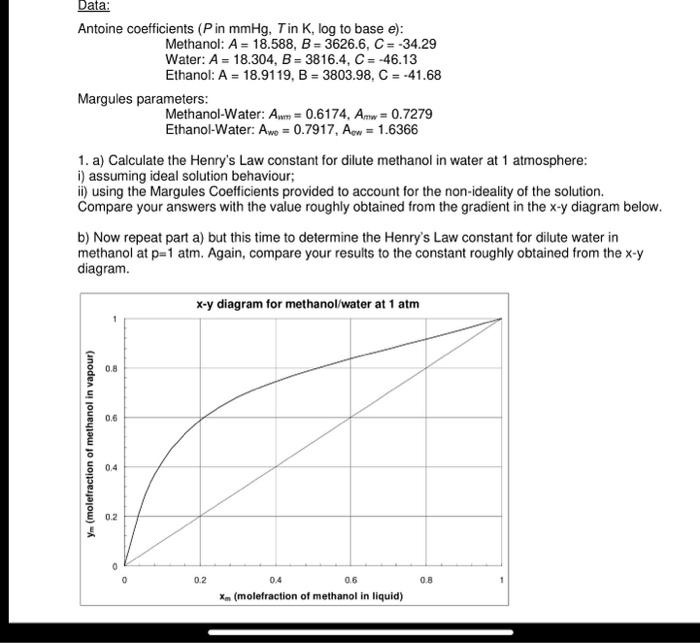
Antoine coefficients ( P in mmHg,T in K, log to base e ): Methanol: A=18.588,B=3626.6,C=34.29 Water: A=18.304,B=3816.4,C=46.13 Ethanol: A=18.9119,B=3803.98,C=41.68 Margules parameters: Methanol-Water: Amm=0.6174,Amw=0.7279 Ethanol-Water: Aw=0.7917,Aww=1.6366 1. a) Calculate the Henry's Law constant for dilute methanol in water at 1 atmosphere: i) assuming ideal solution behaviour; ii) using the Margules Coefficients provided to account for the non-ideality of the solution. Compare your answers with the value roughly obtained from the gradient in the xy diagram below. b) Now repeat part a) but this time to determine the Henry's Law constant for dilute water in methanol at p=1atm. Again, compare your results to the constant roughly obtained from the xy diagram 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


