Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A. One gram (1.00 g) of activated charcoal was added to 100 ml. of the following acetic acid solutions and shaken until equilibrium has
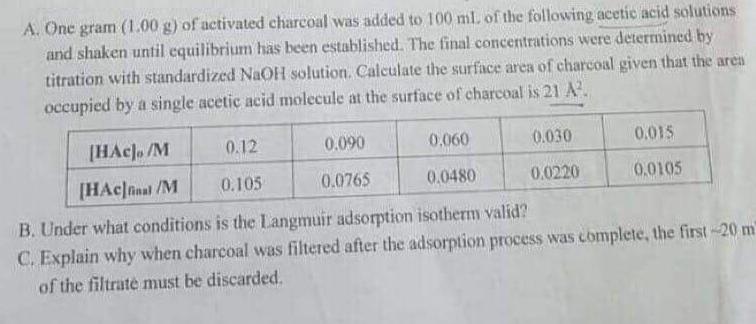
A. One gram (1.00 g) of activated charcoal was added to 100 ml. of the following acetic acid solutions and shaken until equilibrium has been established. The final concentrations were determined by titration with standardized NaOH solution. Calculate the surface area of charcoal given that the aren occupied by a single acetic acid molecule at the surface of charcoal is 21 A. [HAcl. /M 0.12 0.090 0.060 0.030 0.015 [HAcJnal /M 0.105 0.0765 0.0480 0.0220 0,0105 B. Under what conditions is the Langmuir adsorption isotherm valid? C. Explain why when charcoal was filtered after the adsorption process was complete, the first-20 m of the filtrate must be discarded.
Step by Step Solution
★★★★★
3.46 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
B Vlidtin f Lngmuir istherm The Lngmuir istherm will be vlid when the eh tiv...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


