Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Perform a functional group analysis on oxycodone and morphine. How are they the same and how do they differ? note: the only difference I see
Perform a functional group analysis on oxycodone and morphine. How are they the same and how do they differ?
H OH N-CH3 N-CH3 H Oxycodone Hydrocodone note: the only difference I see is the OH molecule on thr oxycodone, but theres just an H molecule on the Hydrocodone. Its hard for me to explain where these functional groups are located. There's 5 cyclohexane molecules, how would I accurately describe the locations? We havent gone over nomenclature on these types of molecules yet. 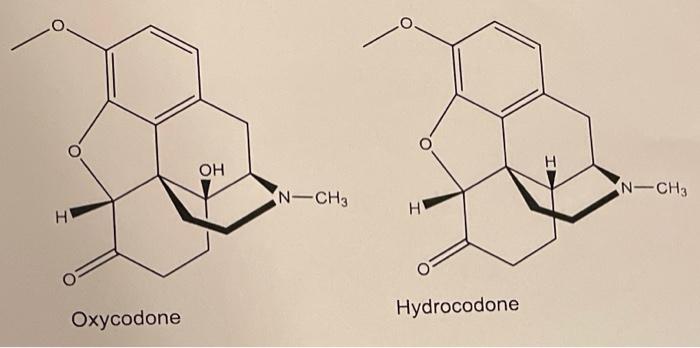

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


