Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Phosgene (COC1) is a toxic substance that forms readily from carbon monoxide and chlorine at elevated temperatures: If 0.450 mol of each reactant is
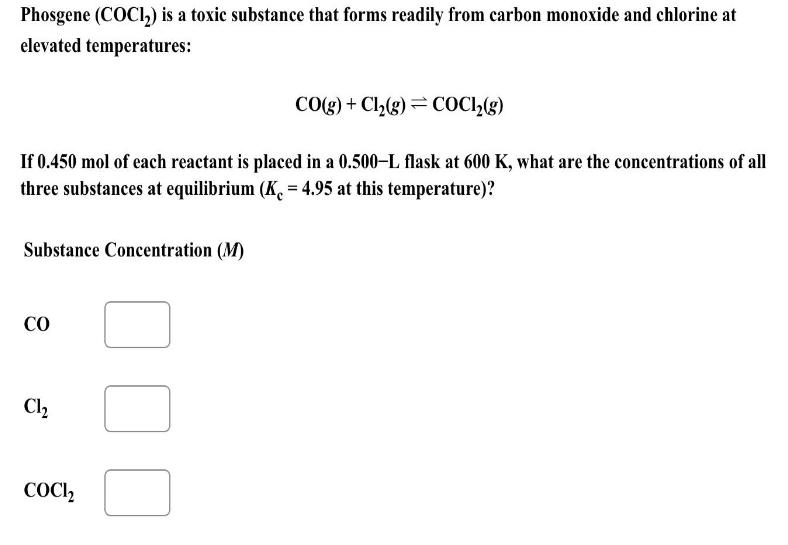
Phosgene (COC1) is a toxic substance that forms readily from carbon monoxide and chlorine at elevated temperatures: If 0.450 mol of each reactant is placed in a 0.500-L flask at 600 K, what are the concentrations of all three substances at equilibrium (K = 4.95 at this temperature)? Substance Concentration (M) CO Cl COCI CO(g) + Cl(g) = COC1(g) J
Step by Step Solution
There are 3 Steps involved in it
Step: 1
1 To solve this problem we can use the given equilibrium constant K and the initial concentrations o...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


