Answered step by step
Verified Expert Solution
Question
1 Approved Answer
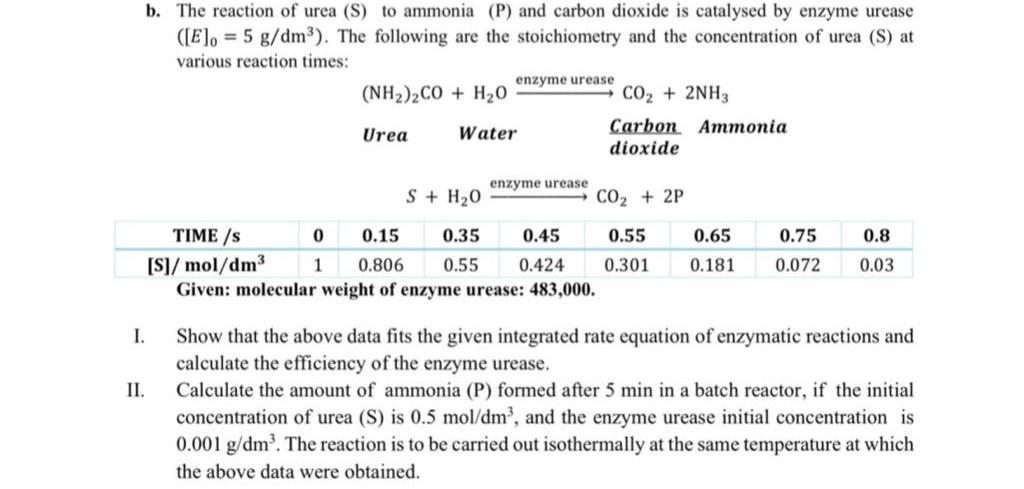
physical chemistry b. The reaction of urea (S) to ammonia (P) and carbon dioxide is catalysed by enzyme urease (El. = 5 g/dm3). The following
physical chemistry
b. The reaction of urea (S) to ammonia (P) and carbon dioxide is catalysed by enzyme urease (El. = 5 g/dm3). The following are the stoichiometry and the concentration of urea (S) at various reaction times: enzyme urease (NH2)2CO + H2O CO2 + 2NH3 Urea Water Carbon Ammonia dioxide enzyme urease S + H20 CO2 + 2P 0.65 0.75 TIME/s 0 0.15 0.35 0.45 0.55 [S]/ mol/dm3 1 0.806 0.55 0.424 0.301 Given: molecular weight of enzyme urease: 483,000. 0.8 0.03 0.181 0.072 1. II. Show that the above data fits the given integrated rate equation of enzymatic reactions and calculate the efficiency of the enzyme urease. Calculate the amount of ammonia (P) formed after 5 min in a batch reactor, if the initial concentration of urea (S) is 0.5 mol/dm?, and the enzyme urease initial concentration is 0.001 g/dm. The reaction is to be carried out isothermally at the same temperature at which the above data were obtainedStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


