Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Physical Chemistry question: a) By using the info in the table draw a phase diagram for the A-B system ( each value in the table
Physical Chemistry question:
T(K) 295 300 305 310 315 320 325 330 335 340 345 (A) Rich phase 4.20 4.36 446 4.67 (grams) 4,893331 6.04 6.94 8,55 11.13 1795 (B) Rich phase 36,12 35.69 34.97 34.14 33.40 32,31 3141 30,14 28.05 24.67 1795 (grams) a) By using the info in the table draw a phase diagram for the A-B system ( each value in the table shows the gram value fo B inside a 50 g solution. All the values given correspond to the concentrations on the phase line) 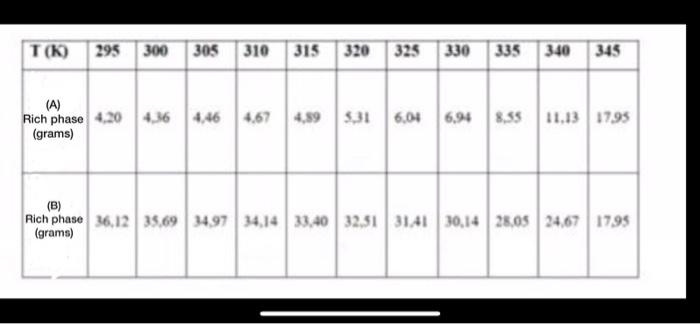
b) In a system containing 65g water and 35 g phenol find the amount of both phases in 310K and find the weight percentage in terms of B in grams
c) When a liquid system of 85 C that contains 60 g B and 40 g A is cooled down, at which temperature can one observe two phases for the first time? Determine the amount of both phases in terms of phenol in grams

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


