Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Physical Chemistry question. Please answer the question clearly in handwriting. Do not use chatgpt and similar applications while solving the question. a) Derive the half
Physical Chemistry question. Please answer the question clearly in handwriting. Do not use chatgpt and similar applications while solving the question. 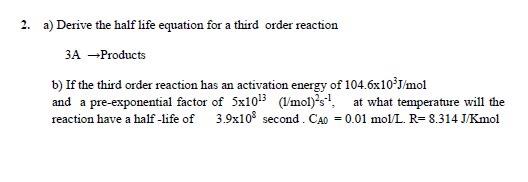
a) Derive the half life equation for a third order reaction 3A Products b) If the third order reaction has an activation energy of 104.6103J/mol and a pre-exponential factor of 51013(1/mol)2s1, at what temperature will the reaction have a half -life of 3.9108 second CA0=0.01mol/R=8.314J/Kmol 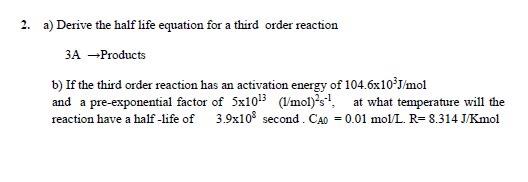
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


