Question: Physics 230 Worksheet Thermal Process Worksheet Part b Comments/Suggestions A diatomic ideal gas with N molecules has an initial pressure p_(0) and initial volume V_(0)
Physics 230 Worksheet\ Thermal Process Worksheet Part b\ Comments/Suggestions\ A diatomic ideal gas with
Nmolecules has an initial pressure
p_(0)and initial volume
V_(0). The gas then undergoes a series of three transformations:\ A Bunsen burner causes the gas to expand, at constant pressure, to a volume
7V_(0).\ The volume is then held constant while an ice bath lowers the pressure to
(p_(0))/(4).\ Finally, a water bath allows the gas to be compressed along a straight line in the
pV-diagram until the pressure and the volume returns to their initial values.\ a. Sketch this cycle of transformation on a
pV-diagram.\ b. Find the temperature at all three "corners" of the cycle. Express these temperatures in terms of
p_(0),
V_(0), and
N.\ c. Find
\\\\Delta U_(i), the change in internal energy of the gas during each of the three processes listed above that make up the cycle. Express your answer in terms of
p_(0), and
V_(0).\ d. What is
\\\\sum_(i=1)^3 \\\\Delta U_(i)for the cycle? Is this reasonable?\ An ideal gas of
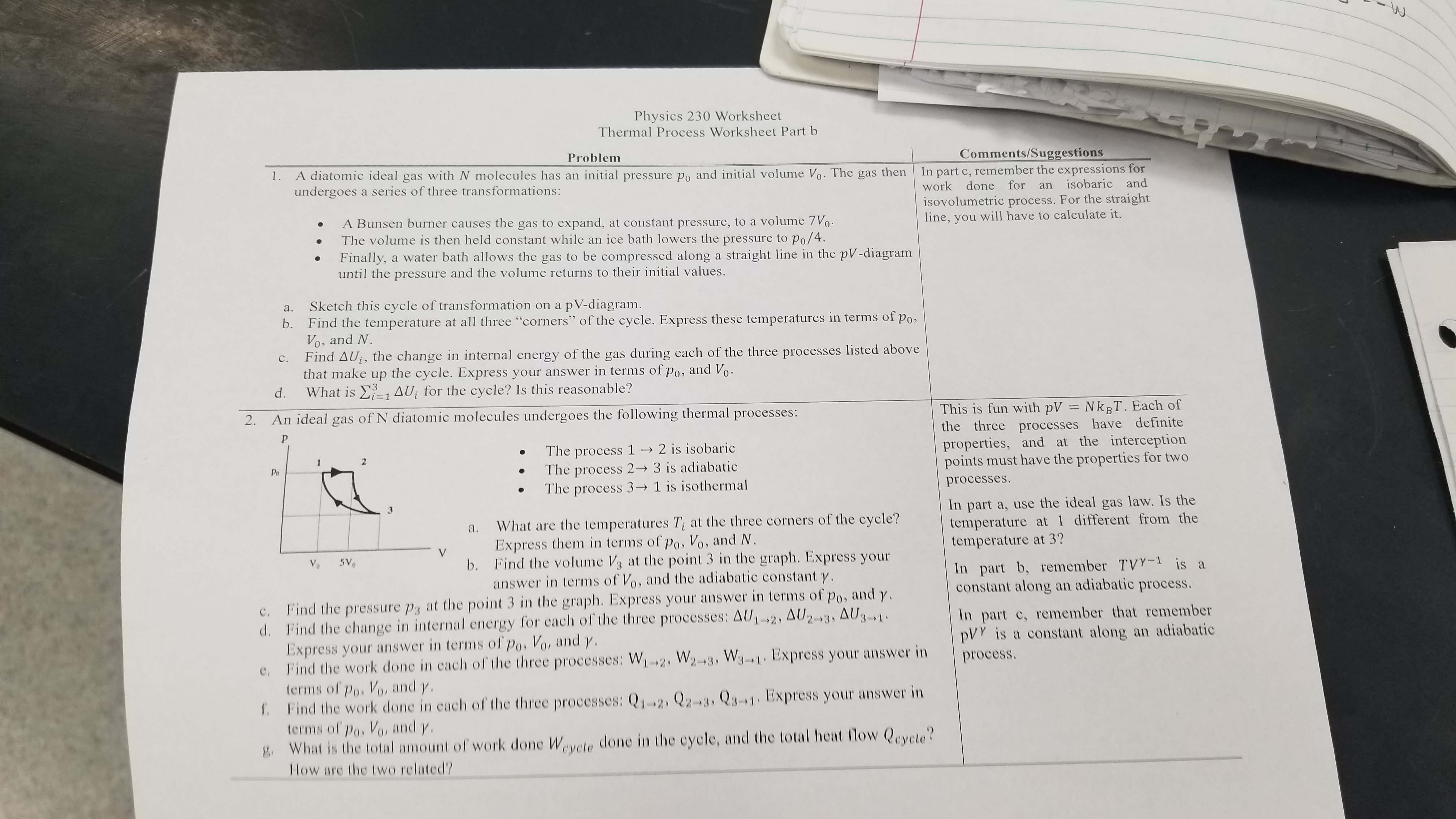
Ndiatomic molecules undergoes the following thermal processes:\ The process
1->2is isobaric\ The process
2->3is adiabatic\ The process
3->1is isothermal\ a. What are the temperatures
T_(i)at the three corners of the cycle? Express them in terms of
p_(0),V_(0), and
N\ b. Find the volume
V_(3)at the point 3 in the graph. Express your answer in terms of
V_(0), and the adiabatic constant
\\\\gamma .\ c. Find the pressure
p_(3)at the point 3 in the graph. Express your answer in terms of
p_(0), and
\\\\gamma .\ d. Find the change in internal energy for each of the three processes:
\\\\Delta U_(1->2),\\\\Delta U_(2->3),\\\\Delta U_(3->1).\ Express your answer in terms of
p_(0),V_(0), and
\\\\gamma .\ e. Find the work done in each of the three processes:
W_(1->2),W_(2->3),W_(3->1). Express your answer in terms of
p_(0),V_(0), and
\\\\gamma .\ Find the work done in each of the three processes:
Q_(1->2),Q_(2->3),Q_(3->1). Express your answer in terms of
p_(0),V_(0), and
\\\\gamma _(. ).\ g. What is the total amount of work done
W_(cyele )done in the cycle, and the total heat flow Qeycle? How are the Iwo related?\ This is fun with
pV=Nk_(B)T. Each of the three processes have definite properties, and at the interception points must have the properties for two processes.\ In part a, use the ideal gas law. Is the temperature at 1 different from the temperature at 3 ?\ In part b, remember
TV^(\\\\gamma -1)is a constant along an adiabatic process.\ In part c, remember that remember
pV^(\\\\gamma )is a constant along an adiabatic process.
Problem A diatomic ideal gas with N molecules has an initial pressure p0 and initial volume V0. The gas then undergoes a series of three transformations: - A Bunsen burner causes the gas to expand, at constant pressure, to a volume 7V0. - The volume is then held constant while an ice bath lowers the pressure to p0/4. - Finally, a water bath allows the gas to be compressed along a straight line in the pV-diagram until the pressure and the volume returns to their initial values. a. Sketch this cycle of transformation on a pV-diagram. b. Find the temperature at all three "corners" of the cycle. Express these temperatures in terms of p0, V0, and N. c. Find Ui, the change in internal energy of the gas during each of the three processes listed above that make up the cycle. Express your answer in terms of p0, and V0. d. What is i=13Ui for the cycle? Is this reasonable? 2. An ideal gas of N diatomic molecules undergoes the following thermal processes: - The process 12 is isobaric - The process 23 is adiabatic - The process 31 is isothermal a. What are the temperatures Ti at the three corners of the cycle? Express them in terms of p0,V0, and N. b. Find the volume V3 at the point 3 in the graph. Express your answer in terms of V0, and the adiabatic constant . c. Find the pressure p3 at the point 3 in the graph. Express your answer in terms of p0, and . d. Find the change in internal energy for each of the three processes: U12,U23,U31. Express your answer in terms of p0,V0, and . c. Find the work done in each of the three processes: W12,W23,W31. Express your answer in terms of p0,V0, and .. 1. Find the work done in each of the three processes: Q12,Q23,Q31. Express your answer in terms of 0,V0, and .. g. What is the total amount of work done Wcyele done in the eycle, and the total heat llow Qeycle? How are the iwo related? work done for an isobaric and isovolumetric process. For the straight line, you will have to calculate it. This is fun with pV=NkBT. Each of the three processes have definite properties, and at the interception points must have the properties for two processes. In part a, use the ideal gas law. Is the temperature at 1 different from the temperature at 3 ? In part b, remember TV1 is a constant along an adiabatic process. In part c, remember that remember pV is a constant along an adiabatic process
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


