Question
A system has four non-degenerate energy levels. The energy levels are E1 = 0, E2 = 1.4 x 10-23 J, E3 = 4.2 x
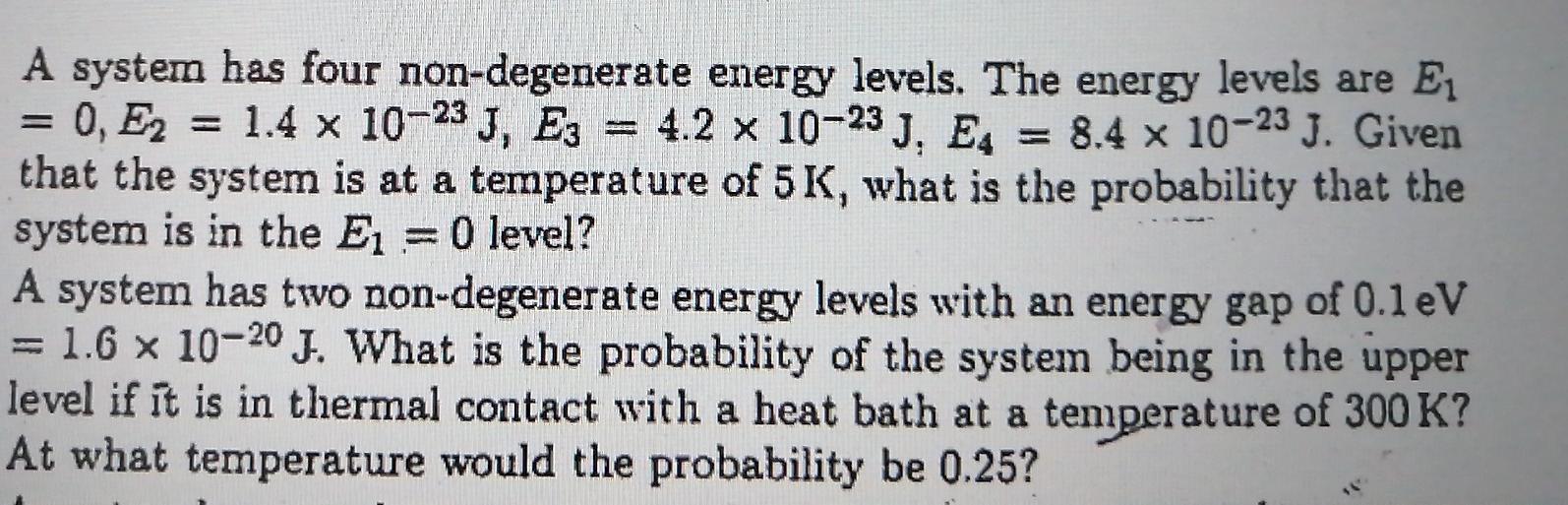
A system has four non-degenerate energy levels. The energy levels are E1 = 0, E2 = 1.4 x 10-23 J, E3 = 4.2 x 10-23 J, E, 8.4 x 10-23 J. Given that the system is at a temperature of 5K, what is the probability that the system is in the E =0 level? A system has two non-degenerate energy levels with an energy gap of 0.leV = 1.6 x 10-20 J. What is the probability of the system being in the upper level if it is in thermal contact with a heat bath at a tenmperature of 300 K? At what temperature would the probability be 0.25? %3D
Step by Step Solution
3.48 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Probability And Statistics For Engineers And Scientists
Authors: Anthony Hayter
3rd Edition
495107573, 978-0495107576
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



