Question
Six grams of liquid X at 35 degrees Celsius are added to 3 grams of liquid Y at 20 degrees Celsius. The specific heat
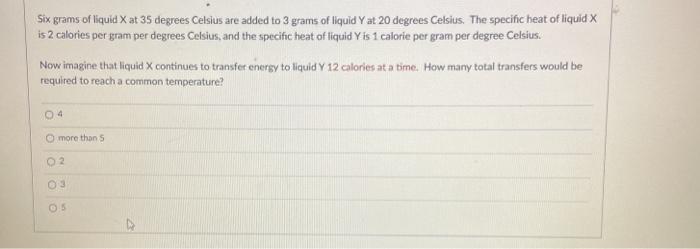
Six grams of liquid X at 35 degrees Celsius are added to 3 grams of liquid Y at 20 degrees Celsius. The specific heat of liquid X is 2 calories per gram per degrees Celsius, and the specific heat of liquid Y is 1 calorie per gram per degree Celsius. Now imagine that liquid X continues to transfer energy to liquid Y 12 calories at a time. How many total transfers would be required to reach a common temperature? 04 O more than S 02
Step by Step Solution
3.50 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
Solution Let the common temperature be t Thus Heat lost ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



