Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Pick the correct statements for the reaction given below. (To theright means the new equilibrium has more product, to the left meansthe new equilibrium has
Pick the correct statements for the reaction given below. (To theright means the new equilibrium has more product, to the left meansthe new equilibrium has more reactant.)
A.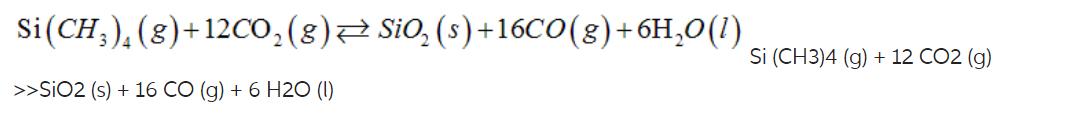
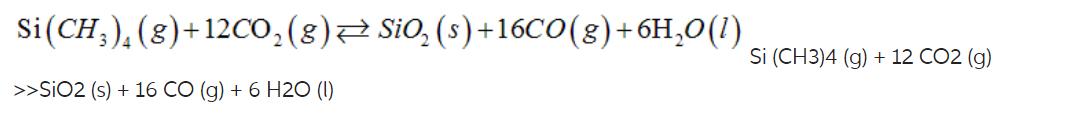
| a. | removing CO2--to the left | |
| b. | removing CO2--no change | |
| c. | removing CO2--to the right | |
| d. | adding CO--tothe left | |
| e. | adding CO--no change | |
| f. | adding CO--to the right | |
| g. | adding SiO2--to the left | |
| h. | adding SiO2--no change | |
| i. | adding SiO2--to the right |
B.
Pick the correct statements for the above reaction. | ||||||||||||||||||||||
| ||||||||||||||||||||||
C.
Pick the correct statements for the above reaction. | ||||||||||||||||||||||
: |
| |||||||||||||||||||||
Si (CH), (g)+12CO (g) SiO (s) +16CO(g) + 6HO(l) >>SIO2 (s) + 16 CO (g) + 6 H20 (1) Si (CH3)4 (g) + 12 CO2 (g)
Step by Step Solution
★★★★★
3.46 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
ANSWER AND STEP BY STEP EXPLANATION The correct statements for the reaction given are a removing CO2to the left b removing H2Oto theleft c expanding the volumeto the left d increasing total pressure by adding an inert gasto the right e increasing total pressure by changing the volumeto the right Explanation In a chemical reaction equilibrium is reached when the concentrations of the reactants and products remain constant When one of the reactants is removed the equilibrium shifts to the left meaning that the reaction produces more of the reactants When one of the products is removed the equilibrium shifts to the right meaning that the reaction produces more of the products Similarly when the volume of the reaction is increased the equilibrium shifts to the left producing more reactants On the other hand when the total pressure of the reaction is increased by adding an inert gas or changing the volume the equilibrium shifts to the right producing more products Therefore in the given reaction removing CO2 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


