Question
pka value of CH3COOH is 4.78. Which of the following statement is correct (A) pH of 0.1 M CH;COOH is 4.78 (B) pH =
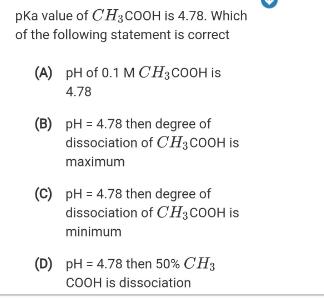
pka value of CH3COOH is 4.78. Which of the following statement is correct (A) pH of 0.1 M CH;COOH is 4.78 (B) pH = 4.78 then degree of dissociation of CH3COOH is maximum (C) pH = 4.78 then degree of dissociation of C'H3COOH is minimum (D) pH = 4.78 then 50% CH3 COOH is dissociation
Step by Step Solution
3.36 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1
p H 7 2 1 p k a p k b pKa is relat...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Applied Linear Algebra
Authors: Peter J. Olver, Cheri Shakiban
1st edition
131473824, 978-0131473829
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



