Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Planck's Constant and Scientific Notation Practice 1. Read the passage 2. Highlight as you read Directions: 3. Solve the 9 questions with your teacher's
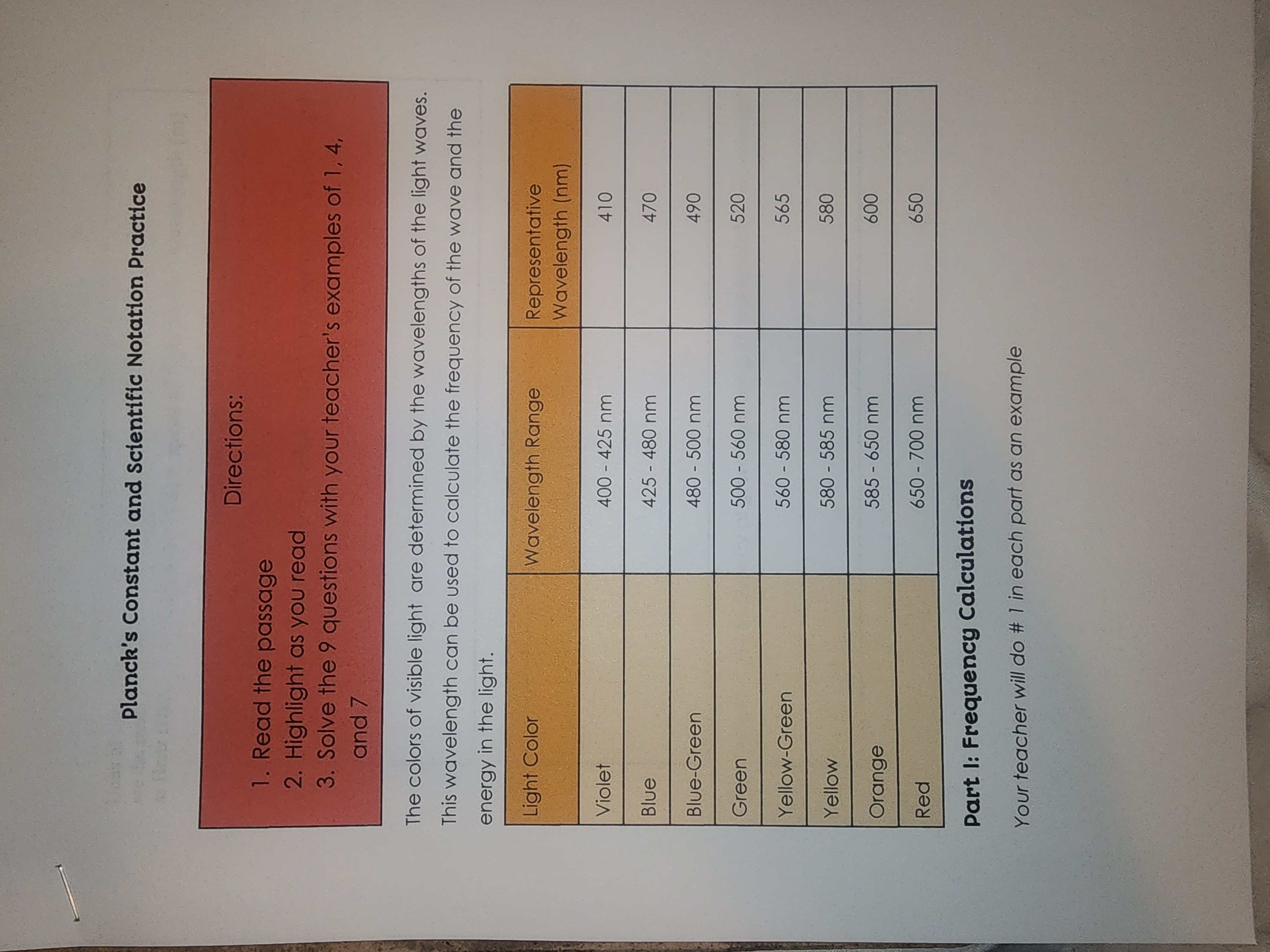


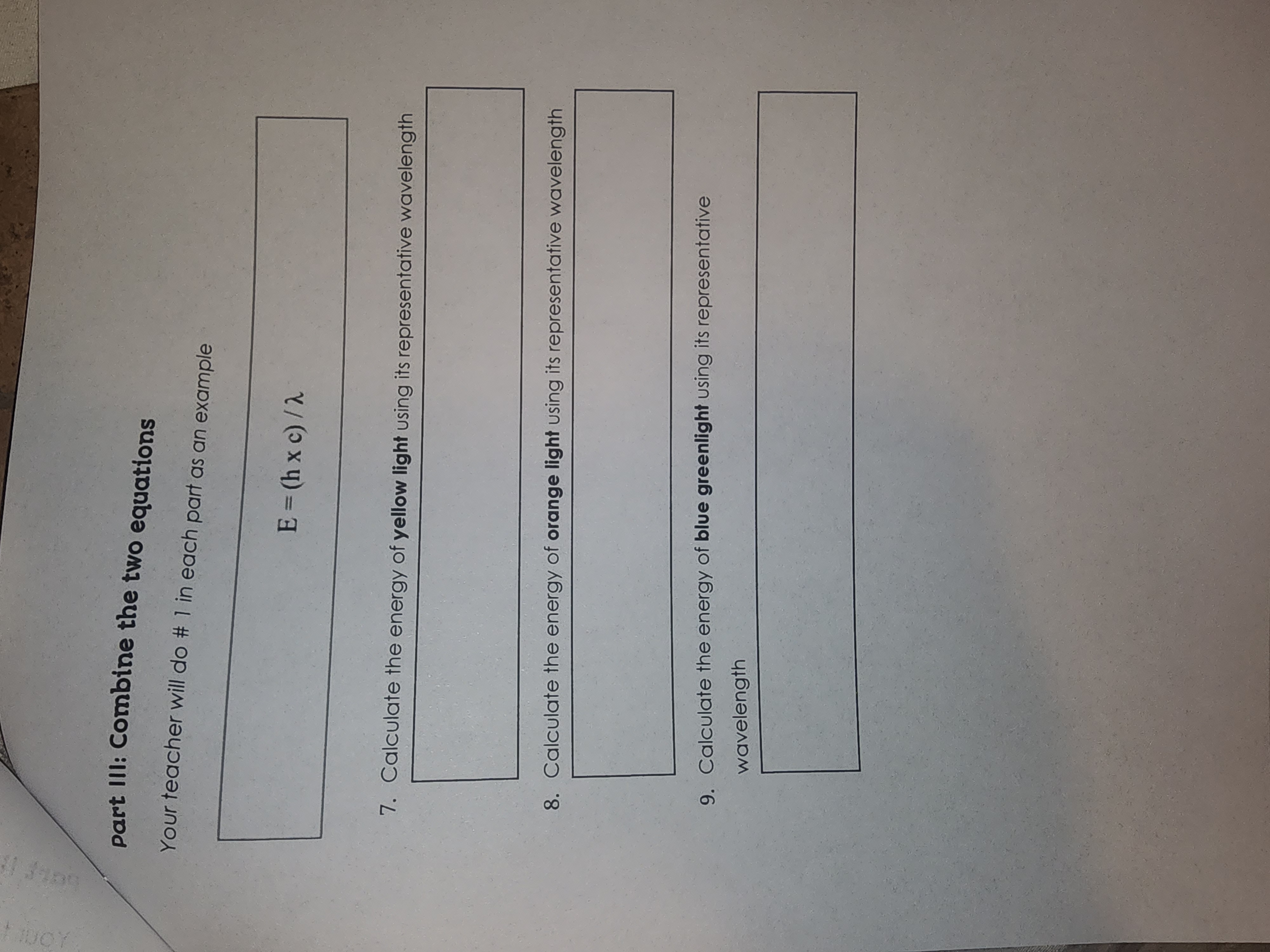
Planck's Constant and Scientific Notation Practice 1. Read the passage 2. Highlight as you read Directions: 3. Solve the 9 questions with your teacher's examples of 1, 4, and 7 The colors of visible light are determined by the wavelengths of the light waves. This wavelength can be used to calculate the frequency of the wave and the energy in the light. Light Color Wavelength Range Representative Wavelength (nm) Violet 400 - 425 nm 410 Blue 425 - 480 nm 470 Blue-Green 480 - 500 nm 490 Green 500 - 560 nm 520 Yellow-Green 560-580 nm 565 Yellow 580 - 585 nm 580 Orange 585 - 650 nm 600 650-700 nm 650 Red Part I: Frequency Calculations Your teacher will do # 1 in each part as an example Units of 1/s are the same as Hertz (Hz) v=c/2 frequency (1/s) = speed of light (m/s) wavelength (m) speed of light = c = 3.00 x 108 m/s 1. Calculate the frequency of blue light using its representative wavelength 2. Calculate the frequency of red light using its representative wavelength 3. Calculate the frequency of green light using its representative wavelength Part II: Energy Calculations Your teacher will do # 1 in each part as an example E=hxv Energy (J) = Planck's constant (J's) x frequency (1/s) Planck's constant = h = 6.63 10-34 J-s 4. Calculate the energy of blue light using the frequency in part I 5. Calculate the energy of red light using the frequency in part I 6. Calculate the energy of green light using the frequency in part part III: Combine the two equations Your teacher will do # 1 in each part as an example E=(hxc)/2 7. Calculate the energy of yellow light using its representative wavelength 8. Calculate the energy of orange light using its representative wavelength 9. Calculate the energy of blue greenlight using its representative wavelength
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


