Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please 5. 50.00 ml of a Mg2+ solution was titrated, to which 10 ml of a buffer solution of pH = 10.0 was added, using
please 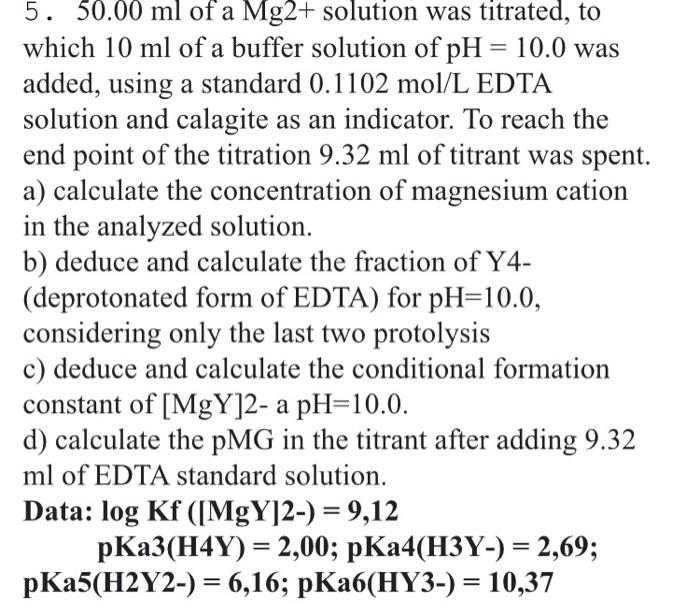
5. 50.00 ml of a Mg2+ solution was titrated, to which 10 ml of a buffer solution of pH = 10.0 was added, using a standard 0.1102 mol/L EDTA solution and calagite as an indicator. To reach the end point of the titration 9.32 ml of titrant was spent. a) calculate the concentration of magnesium cation in the analyzed solution. b) deduce and calculate the fraction of Y4- (deprotonated form of EDTA) for pH=10.0, considering only the last two protolysis c) deduce and calculate the conditional formation constant of [MgY]2- a pH=10.0. d) calculate the PMG in the titrant after adding 9.32 ml of EDTA standard solution. Data: log Kf ([MgY]2-) = 9,12 pKa3(H4Y) = 2,00; pKa4(H3Y-) = 2,69; pKa5(H2Y2-) = 6,16; pKab(HY3-) = 10,37 = = 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


