Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer all 3 questions :) 2/22Al(s)+3CuSO4(aq)Al2(SO4)3(aq)+3Cu(s) 1. How many moles of aluminum sulfate will be produced from 23.4 moles of copper (iI) sulfate? 5.
please answer all 3 questions :) 

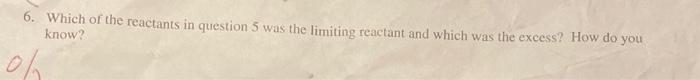
2/22Al(s)+3CuSO4(aq)Al2(SO4)3(aq)+3Cu(s) 1. How many moles of aluminum sulfate will be produced from 23.4 moles of copper (iI) sulfate? 5. If a 16.4gram piece of solid aluminum was reacted with 15.9 graths of 60 pper (II) sulfate in solution. how many grams of copper would form? (Hint: make sure to find the limiting renctant. Show your work!) 6. Which of the reactants in question 5 was the limiting reactant and which was the excess? How do you know 

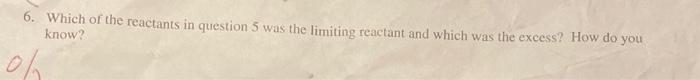
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


