Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer all 4 :) 1. A balloon has a volume of 1.11L at 24C. The balloon is heated to 48.00C. Calculate the new volume
please answer all 4 :) 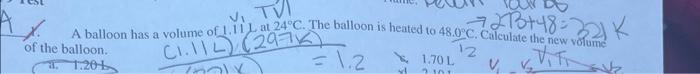
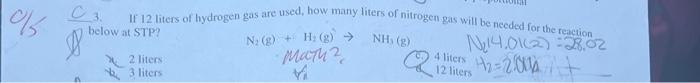
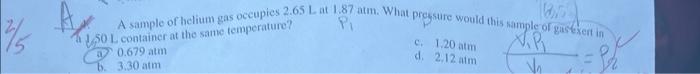

1. A balloon has a volume of 1.11L at 24C. The balloon is heated to 48.00C. Calculate the new volume of the balloon. 3. If 12 liters of hydrogen gas are used, how many liters of nitrogen gas will be needed for the reaction below at STP? N2(g)+H3(g)NH3(g) 2)2liters: Mork? () 4 liters bl 4,0(cm)=0.5.02 .3liters bi 12 liters A sample of helium gas occupies 2.65Lat1.87atm. What pressure would this nample or gisfexert in. (1) 1,50L container at the same temperature? a) 0.679atm c. 1,20atm b. 3.30atm d. 2,12 atm (2.) The pressure of a gas increases when volume decreases A. Solids require heat in order to change into gases d. Some gases only react with each othergat high temperatures 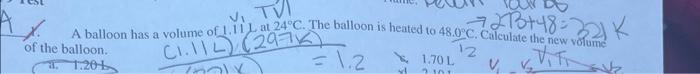
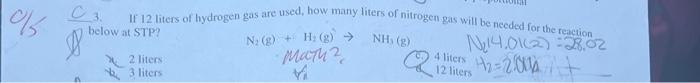
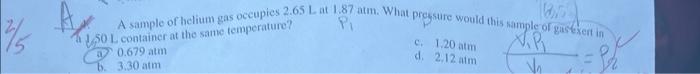

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


