Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer all 4 questions :) 10. How many moles of solute are present in 25.0mL of 2.00MHCl ? a. 0.0800mol c. 50.0molCl=3O.4S b. 0.0500mol
please answer all 4 questions :) 
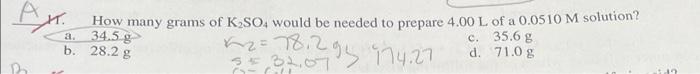

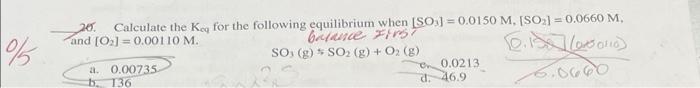
10. How many moles of solute are present in 25.0mL of 2.00MHCl ? a. 0.0800mol c. 50.0molCl=3O.4S b. 0.0500mol d. 2.00mol 11. How many grams of K2SO4 would be needed to prepare 4.00L of a 0.0510M solution? a. 34.5g x2=78.29 c 74.27 c. 35.6g d. 71.0g 12. What volume of 18.0M sulfuric acid is required to prepare 16.5L of 0.126M sulfuric acid? a. 0.264L b. 0.137L c. 0.116L d. 0.232L 26. Calculate the Kcq for the following equilibrium when [SO3]=0.0150M,[SO2]=0.0660M, and [O2]=0.00110M. a. 0.00735 SO3(g)SO2(g)+O2(g) b. 136 d. 46.9 
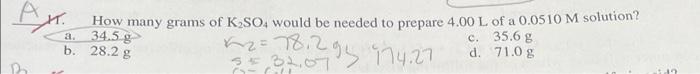

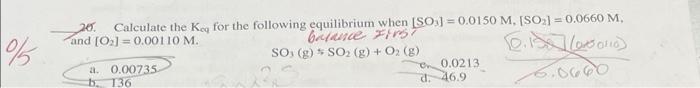
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


