Answered step by step
Verified Expert Solution
Question
1 Approved Answer
PLEASE ANSWER ALL i will give thumbs up if answered allll please i need help 1. Which of the Newman projections (a or b) is
PLEASE ANSWER ALL 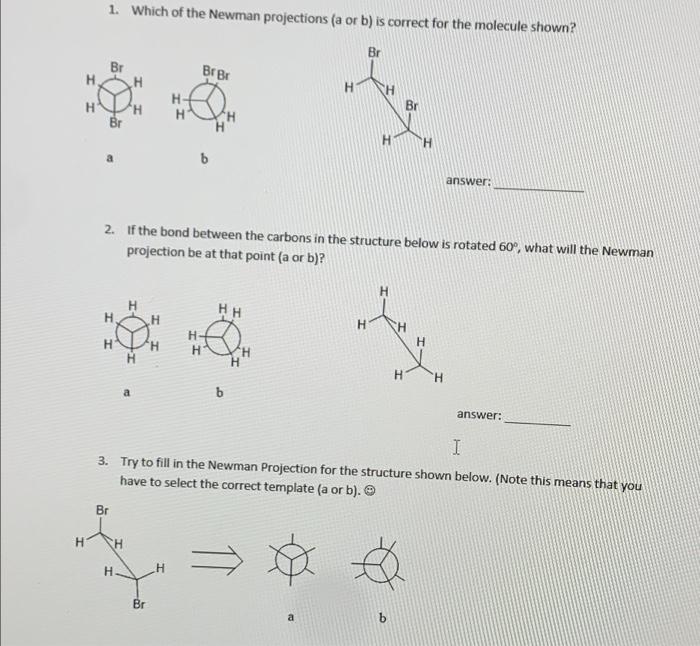
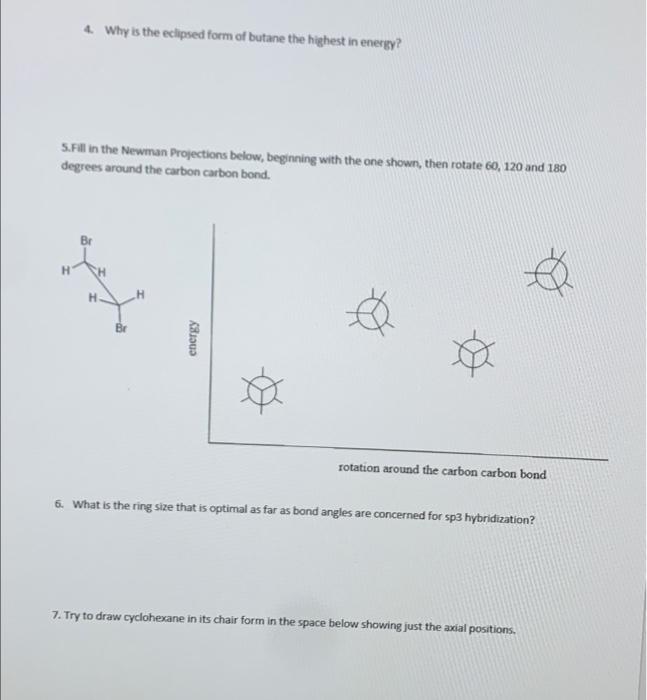

1. Which of the Newman projections (a or b) is correct for the molecule shown? Br Br Br H H nero H- H Br H Br H 6 answer: 2. If the bond between the carbons in the structure below is rotated 60, what will the Newman projection be at that point (a or b)? H H HH H H H H H- H H 6 answer: I 3. Try to fill in the Newman Projection for the structure shown below. (Note this means that you have to select the correct template (a or b). Br H CH H H Br a 4 Why is the eclipsed form of butane the highest in energy? 5.Fill in the Newman Projections below, beginning with the one shown, then rotate 60, 120 and 180 degrees around the carbon carbon bond. Br H H Br energy rotation around the carbon carbon bond 6. What is the ring size that is optimal as far as bond angles are concerned for sp3 hybridization? 7. Try to draw cyclohexane in its chair form in the space below showing just the axial positions. 8. Draw cyclohexane in the chair form in the space below showing just the equatorial positions 9. Why are the equatorial position generally preferred (energy wise) over the axial positions? 10. Which is lower in energy, cis or trans 1,3-difluoro cyclohexane? Draw the structure in the space below. 11. Which is more stable, a or b? Why? answer: b i will give thumbs up if answered allll please i need help



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


