Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer all parts and show work!! thank you so much 1. The vapor pressure of an unknown liquid was measured as described in this
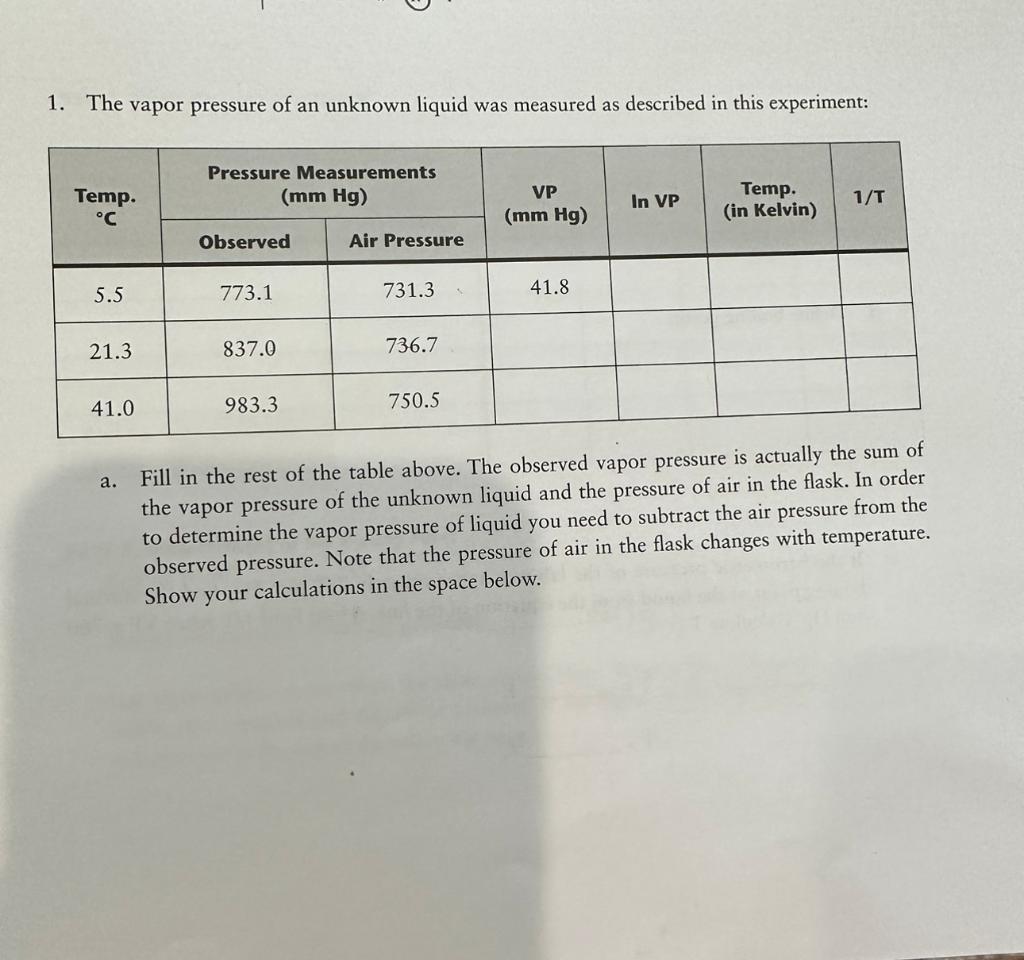

please answer all parts and show work!! thank you so much
1. The vapor pressure of an unknown liquid was measured as described in this experiment: a. Fill in the rest of the table above. The observed vapor pressure is actually the sum of the vapor pressure of the unknown liquid and the pressure of air in the flask. In order to determine the vapor pressure of liquid you need to subtract the air pressure from the observed pressure. Note that the pressure of air in the flask changes with temperature. Show your calculations in the space below. Vapor Pressure and Heat of Vaporization of Liquids b. Using Excel or Google Sheets, graph ln VP vs. 1/T. Let ln VP be the ordinate (vertical axis). Calculate the best straight line through the points on the graph. c. Use the equation of the line to determine the molar heat of vaporization. Show your calculations in the space below. Slope=Hvap/R(Hvap=JapJ/mol) d. Define boiling point: e. If the barometric pressure of the lab was approximately 760mmHg, find the normal boiling point of the liquid from the equation of the line. (Hint: Find 1/T when VP=760 mmHg. Calculate T in C.) 1/T=T=KT=CStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


