Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer all parts fully and show all work, thank you so much! 1. (a) Determine the enthalpy of combustion of C3H6:2C3H6(g)+O2(g)CO2(g)+H2O (I) from the
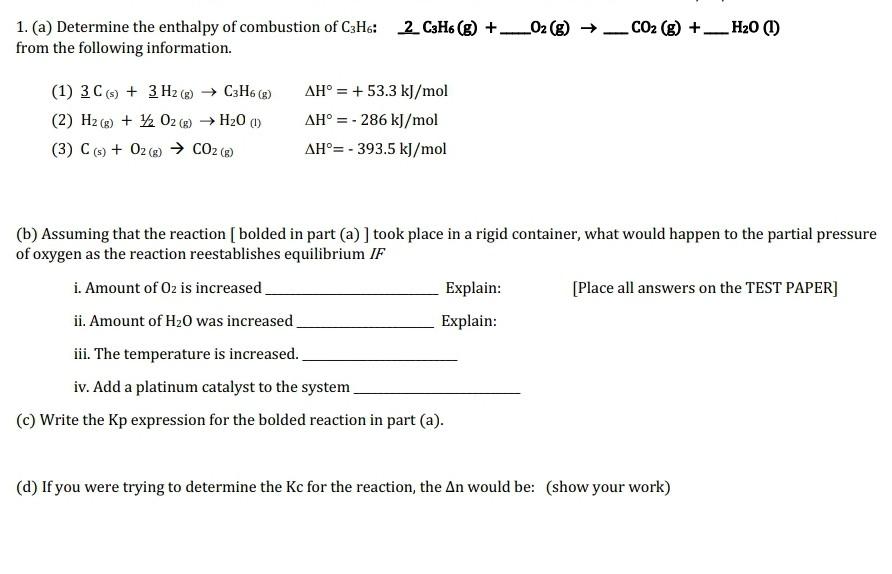
please answer all parts fully and show all work, thank you so much!
1. (a) Determine the enthalpy of combustion of C3H6:2C3H6(g)+O2(g)CO2(g)+H2O (I) from the following information. (1) 3C(s)+3H2(g)C3H6(g)H=+53.3kJ/mol (3) C(s)+O2(g)CO2(g)H=393.5kJ/mol (b) Assuming that the reaction [bolded in part (a) ] took place in a rigid container, what would happen to the partial pressure of oxygen as the reaction reestablishes equilibrium IF i. Amount of O2 is increased Explain: [Place all answers on the TEST PAPER] ii. Amount of H2O was increased Explain: iii. The temperature is increased. iv. Add a platinum catalyst to the system (c) Write the Kp expression for the bolded reaction in part (a). (d) If you were trying to determine the Kc for the reaction, the n would be: (show your work)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


