Question
Please answer all parts, is all one question. Assuming that layer silicates and humic acid exist independently in the soil, calculate a typical CEC that
Please answer all parts, is all one question.
Assuming that layer silicates and humic acid exist independently in the soil, calculate a typical CEC that would be measured for a soil at pH 7 containing
Example to answer the next parts for the same question:
CECsoil= (Fclay * CECclay) + (Forganic matter * CECorganic matter)= ((300g/1000g)(1mol*kg^-1))+((20g/1000g)(1.6mol*kg^-1))= 0.332mol/kg
(a) 270 g kg-1 montmorillonite and 25 g kg-1 SOM,
(b) 270 g kg-1 kaolinite and 25 g kg-1 SOM, and
(c) 160 g kg-1 kaolinite and 15 g kg- 1 SOM.
(d) Are these calculations realistic? Why or why not? (See Figure 3 for reasonable values of SOM charge at pH 7).
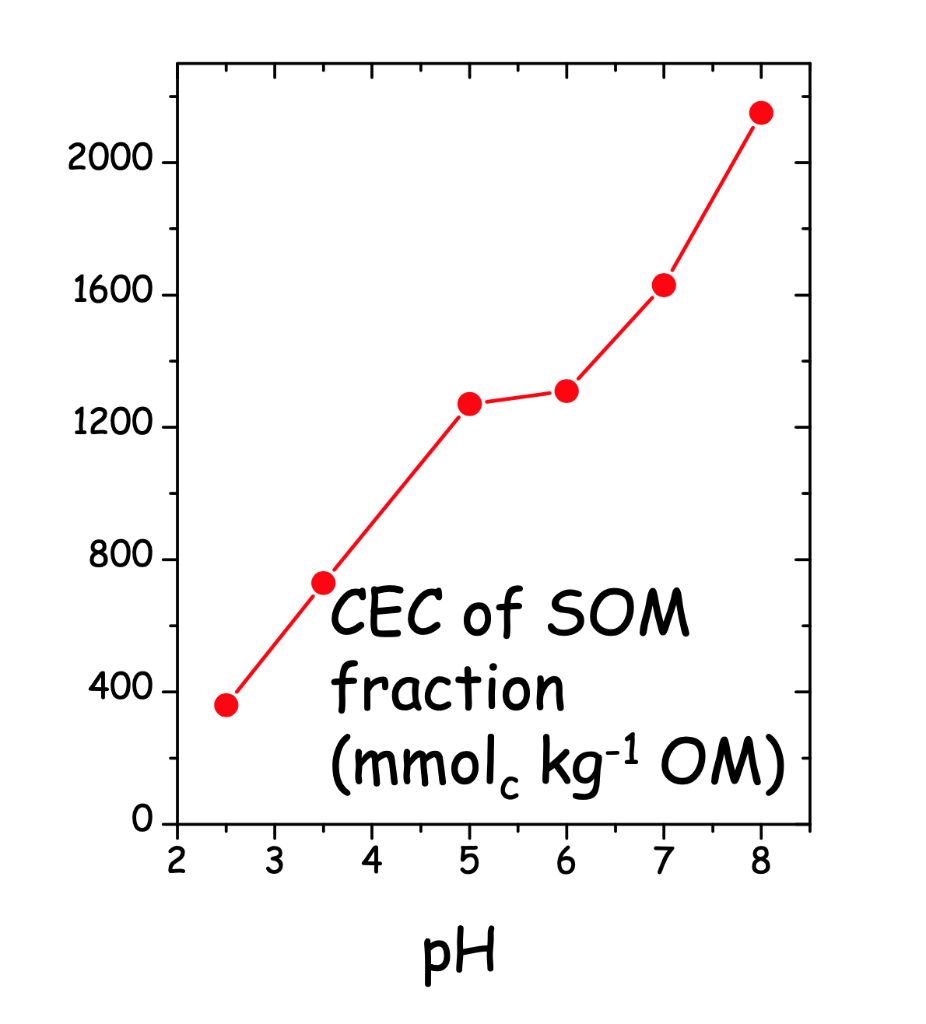
The CEC would be the sum of the structural (permanent) charge in the clay mineral plus that resulting from pH-dependent charge of the SOM.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


