Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer all question thank you :) 3. To what temperature will a 50.0g piece of glass raise if it absorbs 5275 joules of heat
please answer all question thank you :) 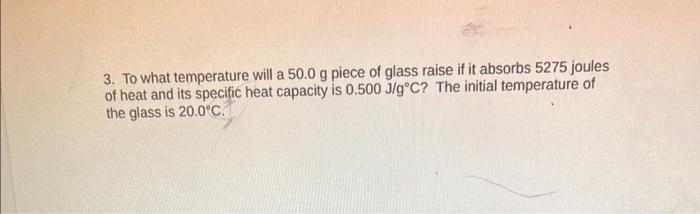



3. To what temperature will a 50.0g piece of glass raise if it absorbs 5275 joules of heat and its specific heat capacity is 0.500J/gC ? The initial temperature of the glass is 20.0C. 4. Calculate the heat capacity of a piece of wood if 1500.0g of the wood absorbs 6.75104 joules of heat, and its temperature changes from 32.0C to 57.0C 5. 100.0mL of 4.00C water is heated until its temperature is 37.0C. If the specific heat of water is 4.184J/gC, calculate the amount of heat energy needed to cause this rise in temperature. 6. 25.0g of mercury is heated from 25.0C to 155.0C, and absorbs 455 joules of heat in the process. Calculate the specific heat capacity of mercury 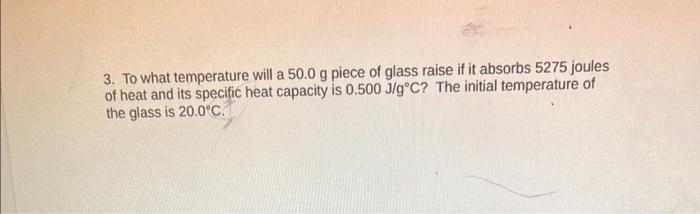



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


