Answered step by step
Verified Expert Solution
Question
1 Approved Answer
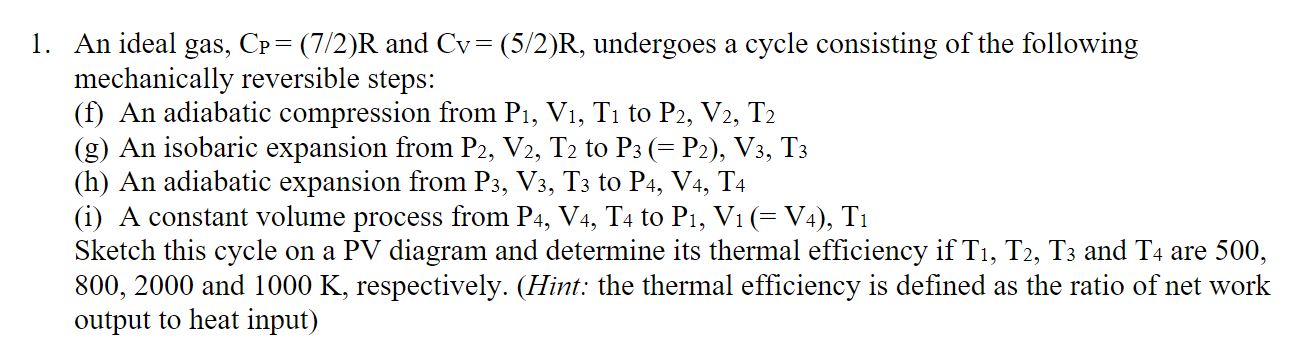
please answer all questions.An ideal gas, C P P = ( 7 2 ) R and C V V = ( 5 2 ) R
please answer all questions.An ideal gas, and undergoes a cycle consisting of the following
mechanically reversible steps:
f An adiabatic compression from to
g An isobaric expansion from to
h An adiabatic expansion from to
i A constant volume process from to
Sketch this cycle on a PV diagram and determine its thermal efficiency if and are
and respectively. Hint: the thermal efficiency is defined as the ratio of net work
output to heat input
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


