Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answer all the above questions correctly providing necessary formulae, equations, expressions, diagrams, charts, graphs, plots and tables. 9. A catalytic reaction AR is made
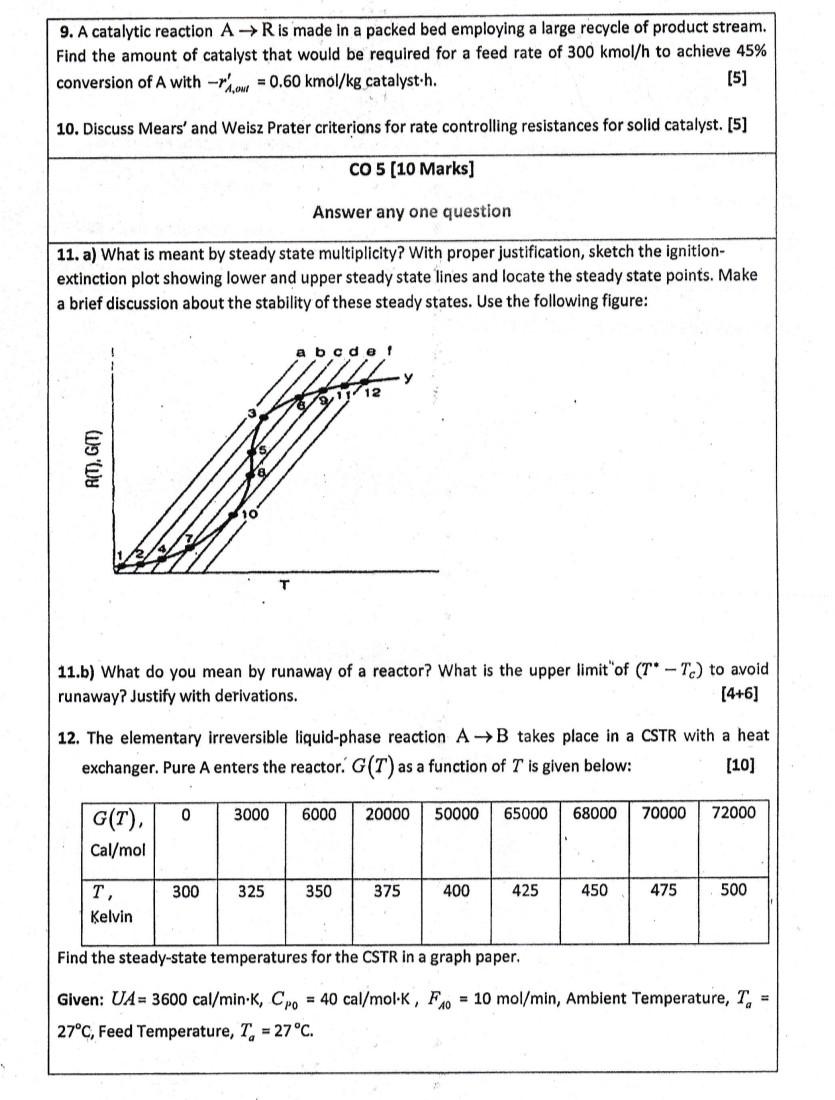
Please answer all the above questions correctly providing necessary formulae, equations, expressions, diagrams, charts, graphs, plots and tables.
9. A catalytic reaction AR is made in a packed bed employing a large recycle of product stream. Find the amount of catalyst that would be required for a feed rate of 300kmol/h to achieve 45% conversion of A with rA,owt=0.60kmol/kg catalyst.h. [5] 10. Discuss Mears' and Weisz Prater criterions for rate controlling resistances for solid catalyst. [5] CO5[10Marks] Answer any one question 11. a) What is meant by steady state multiplicity? With proper justification, sketch the ignitionextinction plot showing lower and upper steady state lines and locate the steady state points. Make a brief discussion about the stability of these steady states. Use the following figure: 11.b) What do you mean by runaway of a reactor? What is the upper limit" of (TTc) to avoid runaway? Justify with derivations. [4+6] 12. The elementary irreversible liquid-phase reaction AB takes place in a CSTR with a heat exchanger. Pure A enters the reactor. G(T) as a function of T is given below: [10] Find the steady-state temperatures for the CSTR in a graph paper. Given: UA=3600cal/minK,CP0=40cal/molK,FA0=10mol/min, Ambient Temperature, Ta= 27C, Feed Temperature, Ta=27CStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


