Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answer b and c Q1. [25 marks] (a) A homogeneous reaction between A and B is occurring within an organic solution. An ideal CFSTR
Please answer b and c
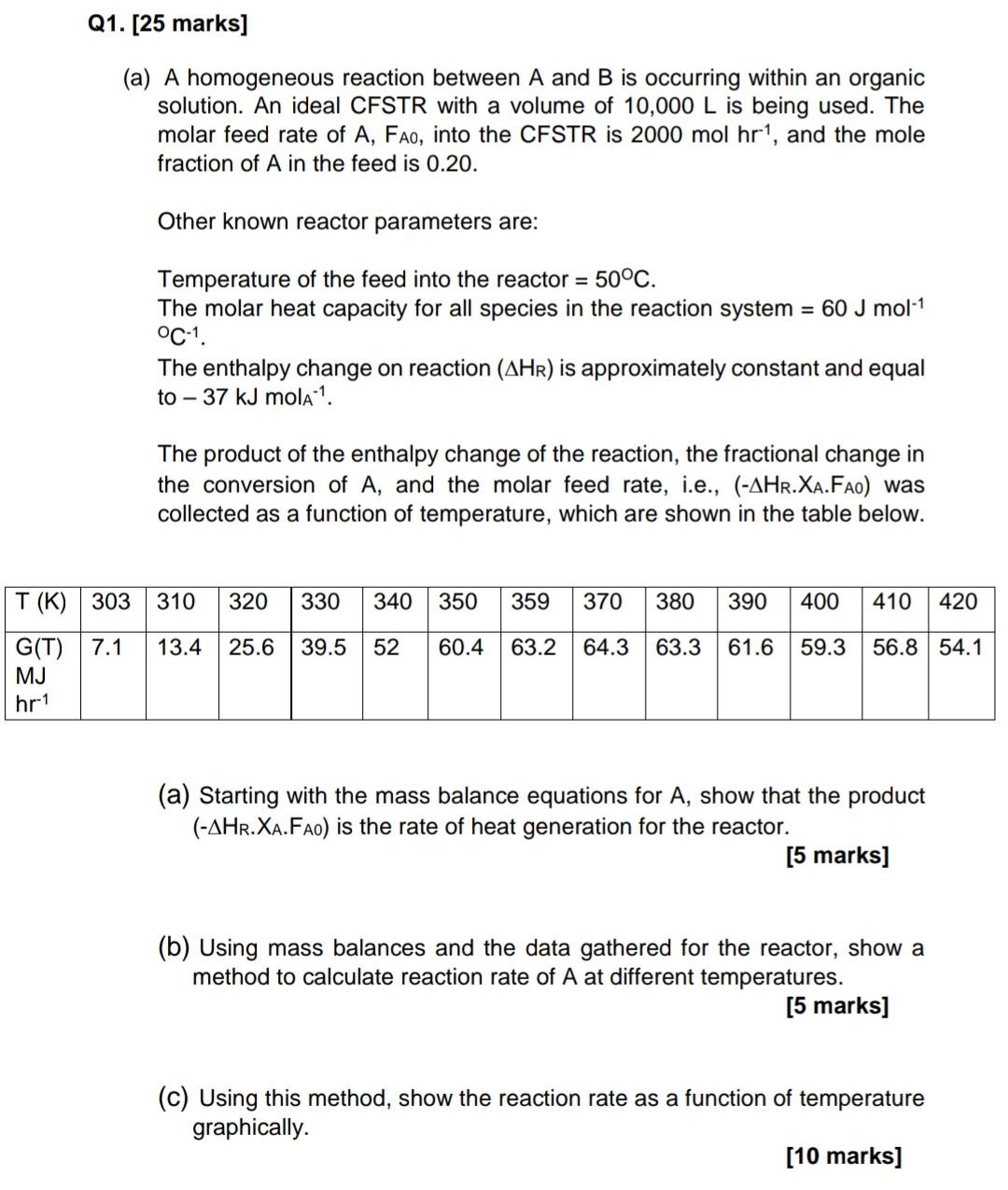
Q1. [25 marks] (a) A homogeneous reaction between A and B is occurring within an organic solution. An ideal CFSTR with a volume of 10,000 L is being used. The molar feed rate of A, Fao, into the CFSTR is 2000 mol hr", and the mole fraction of A in the feed is 0.20. Other known reactor parameters are: Temperature of the feed into the reactor = 50C. The molar heat capacity for all species in the reaction system = 60 J mol-1 C-1 The enthalpy change on reaction (AHR) is approximately constant and equal to - 37 kJ mola 1. The product of the enthalpy change of the reaction, the fractional change in the conversion of A, and the molar feed rate, i.e., (-AHR.XA.Fao) was collected as a function of temperature, which are shown in the table below. T(K) 303 310 320 330 340 350 359 370 380 390 400 410 420 13.4 25.6 39.5 52 60.4 63.2 64.3 63.3 61.6 59.3 56.8 54.1 G(T) 7.1 MJ hr-1 (a) Starting with the mass balance equations for A, show that the product (-AHR.XA.Fao) is the rate of heat generation for the reactor. [5 marks] (b) Using mass balances and the data gathered for the reactor, show a method to calculate reaction rate of A at different temperatures. [5 marks] (C) Using this method, show the reaction rate as a function of temperature graphically. [10 marks]
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


