Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answer BOTH these questions. Thank you. 1. Consider the reaction mechanism for the dimerization of the molecule below. Answer the questions that follow. It
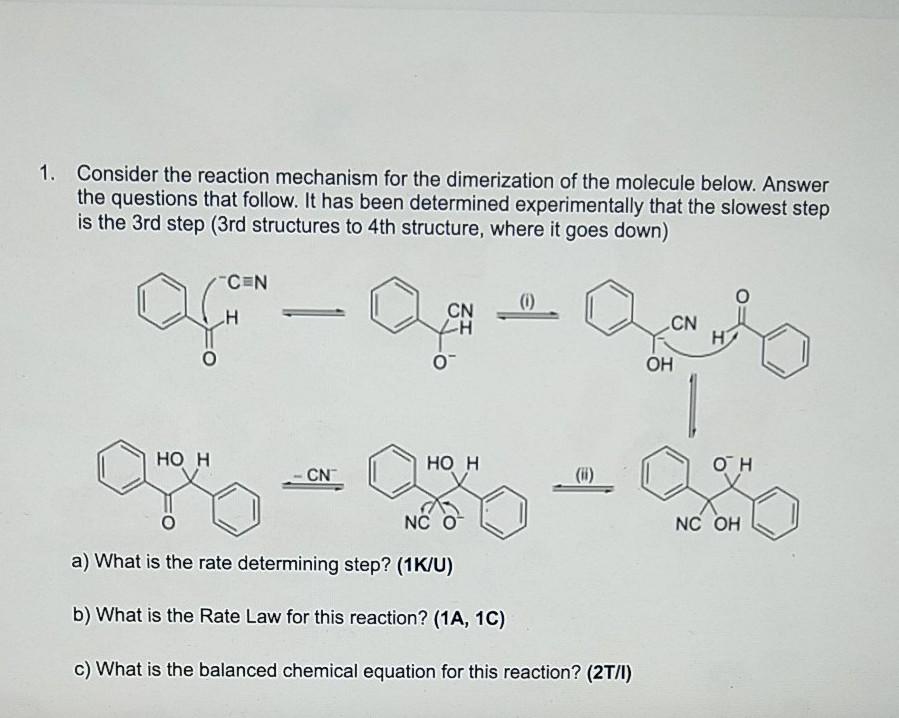
Please answer BOTH these questions. Thank you.
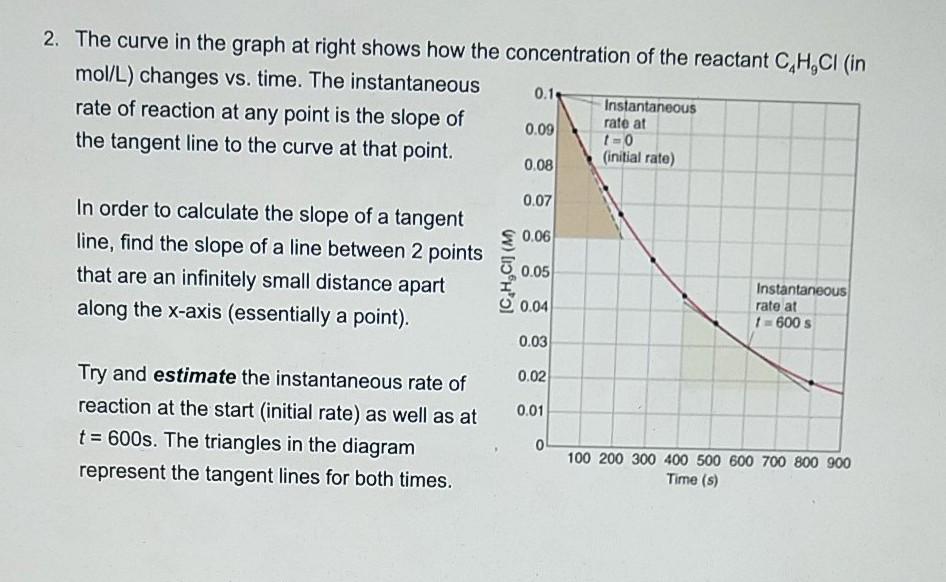
1. Consider the reaction mechanism for the dimerization of the molecule below. Answer the questions that follow. It has been determined experimentally that the slowest step is the 3rd step (3rd structures to 4th structure, where it goes down) 0 CN - CN . OH HO H HOH OH -CN (6) NC O NC OH a) What is the rate determining step? (1K/U) b) What is the Rate Law for this reaction? (1A, 1C) c) What is the balanced chemical equation for this reaction? (27/1) 2. The curve in the graph at right shows how the concentration of the reactant C,H,CI (in mol/L) changes vs. time. The instantaneous 0.1 Instantaneous rate of reaction at any point is the slope of 0.09 rate at 10 the tangent line to the curve at that point. (initial rate) 0.08 0.07 $ 0.06 In order to calculate the slope of a tangent line, find the slope of a line between 2 points that are an infinitely small distance apart along the x-axis (essentially a point). 0.05 0.04 Instantaneous rate at 1600 0.03 0.02 0.01 Try and estimate the instantaneous rate of reaction at the start (initial rate) as well as at t = 600s. The triangles in the diagram represent the tangent lines for both times. 0 100 200 300 400 500 600 700 800 900 Time (s)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


