Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answer each question, I would high appreciate it and upvote aswell. Thanks. 1. Calculate the molar entropy of a rubber. 2. Calculate the temperature
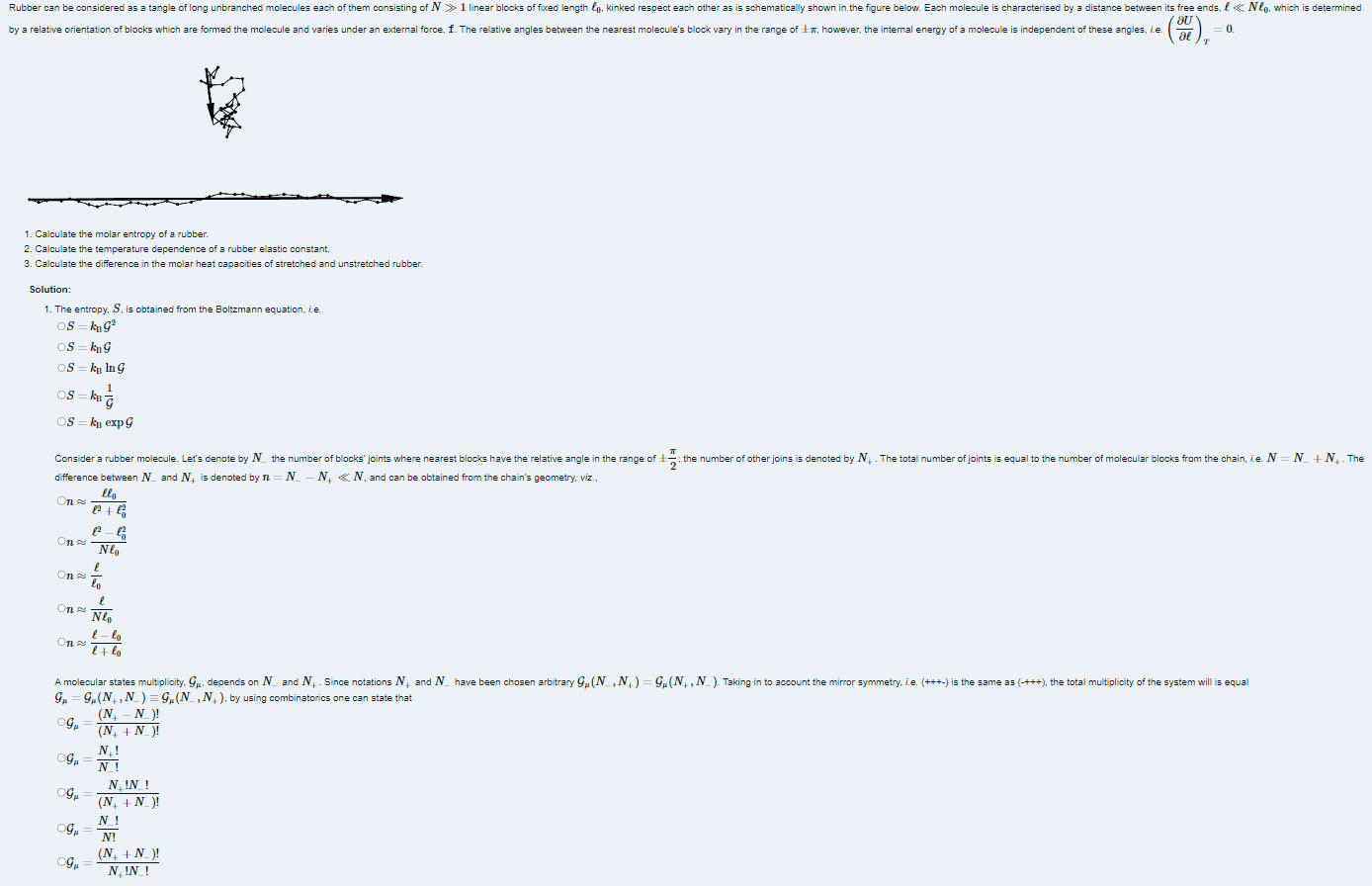
Please answer each question, I would high appreciate it and upvote aswell. Thanks.
1. Calculate the molar entropy of a rubber. 2. Calculate the temperature dependence of a rubber elastic constant. 3. Calculate the difference in the molar heat capacities of stretched and unstretched rubber. Solution: 1. The entropy, S, is obtained from the Boltzmann equation, i.e. S=kBG2S=kBGS=kBlnGS=kBG1S=kBexpG difference between Nand N+is denoted by n=NN+&N, and can be obtained from the chain's geometry, viz., nnnnn2+020N02020N0+00 G=G(N+,N)G(N,N+), by using combinatorics one can state that GGGGG=(N++N)!(N+N)!=N!N+!=(N++N)!N+!N!=N!N!=N+!N!(N++N)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


