Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answer f, g and h Consider the the dx2y2 orbital, which has the angular form (ignoring normalization constants) dx2y2=sin2cos2 f. In what direction(s) is
Please answer f, g and h 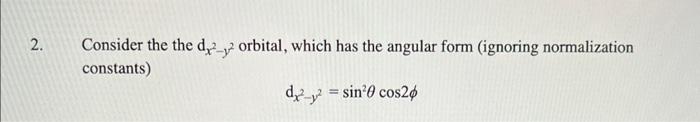
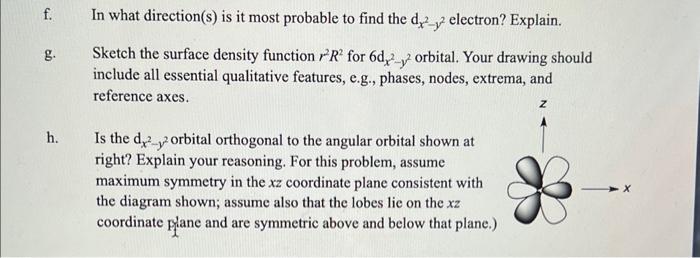
Consider the the dx2y2 orbital, which has the angular form (ignoring normalization constants) dx2y2=sin2cos2 f. In what direction(s) is it most probable to find the dx2y2 electron? Explain. g. Sketch the surface density function r2R2 for 6dx2y2 orbital. Your drawing should include all essential qualitative features, e.g., phases, nodes, extrema, and reference axes. h. Is the dx2y2 orbital orthogonal to the angular orbital shown at right? Explain your reasoning. For this problem, assume maximum symmetry in the xz coordinate plane consistent with the diagram shown; assume also that the lobes lie on the xz coordinate plane and are symmetric above and below that plane.) 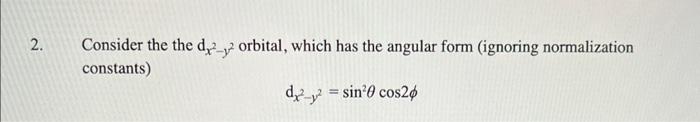

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


