Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer in detail it has more marks so please look at the question tharally The determination of the density of a fluid has many
please answer in detail it has more marks so please look at the question tharally 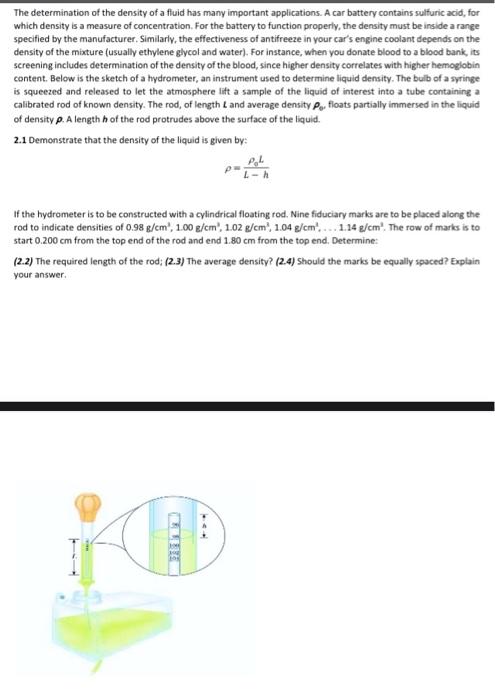
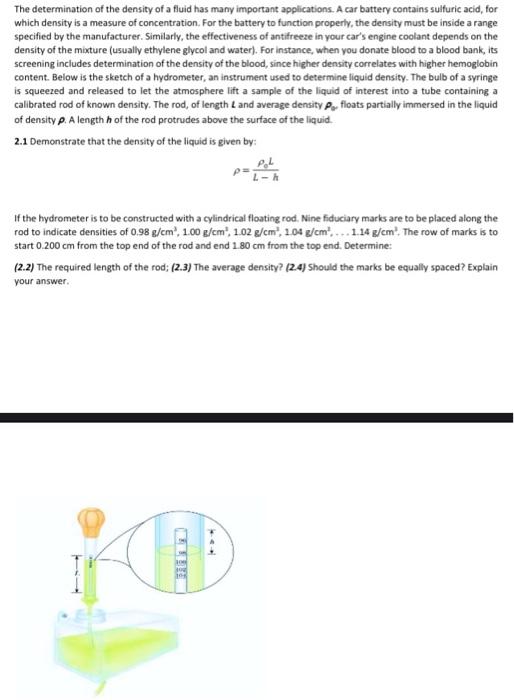
The determination of the density of a fluid has many important applications. A car battery contains sulfuric acid, for which density is a measure of concentration. For the battery to function properly, the density must be inside a range specified by the manufacturer. Similarly, the effectiveness of antifreeze in your car's engine coolant depends on the density of the mixture (usually ethylene glycol and water). For instance, when you donate blood to a blood bank its screening includes determination of the density of the blood, since higher density correlates with higher hemoglobin content. Below is the sketch of a hydrometer, an instrument used to determine liquid density. The bulb of a syringe is squeezed and released to let the atmosphere lift a sample of the liquid of interest into a tube containing a calibrated rod of known density. The rod, of length and average density P. floats partially immersed in the liquid of density p. A length of the rod protrudes above the surface of the liquid. 2.1 Demonstrate that the density of the liquid is given by: If the hydrometer is to be constructed with a cylindrical floating rod. Nine fiduciary marks are to be placed along the rod to indicate densities of 0.98 g/cm', 1.00 g/cm', 1.02 g/cm , 1.04 g/cm'.... 1.14 g/cm'. The row of marks is to start 0.200 cm from the top end of the rod and end 1.80 cm from the top end. Determine: (2.2) The required length of the rod; (2.3) The average density? (2.4) Should the marks be equally spaced? Explain your answer. The determination of the density of a fluid has many important applications. A car battery contains sulfuric acid, for which density is a measure of concentration. For the battery to function properly, the density must be inside a range specified by the manufacturer. Similarly, the effectiveness of antifreeze in your car's engine coolant depends on the density of the mixture (usually ethylene glycol and water). For instance, when you donate blood to a blood bank, its screening includes determination of the density of the blood, since higher density correlates with higher hemoglobin content. Below is the sketch of a hydrometer, an instrument used to determine liquid density. The bulb of a syringe is squeezed and released to let the atmosphere lift a sample of the liquid of interest into a tube containing a calibrated rod of known density. The rod, of length and average density P. floats partially immersed in the liquid of density p. A length of the rod protrudes above the surface of the liquid 2.1 Demonstrate that the density of the liquid is given by: If the hydrometer is to be constructed with a cylindrical floating rod. Nine Fiduciary marks are to be placed along the rod to indicate densities of 0.98 g/cm', 1.00 g/cm", 1.02 g/cm , 104 g/cm, ... 1.14 g/cm'. The row of marks is to start 0.200 cm from the top end of the rod and end 180 cm from the top end. Determine: (2.2) The required length of the rod; (2.3) The average density? (24) Should the marks be equally spaced? Explain your 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


