please answer question 4. i included pictures of the introduction for background. if correct I will thumbs up. thanks you!

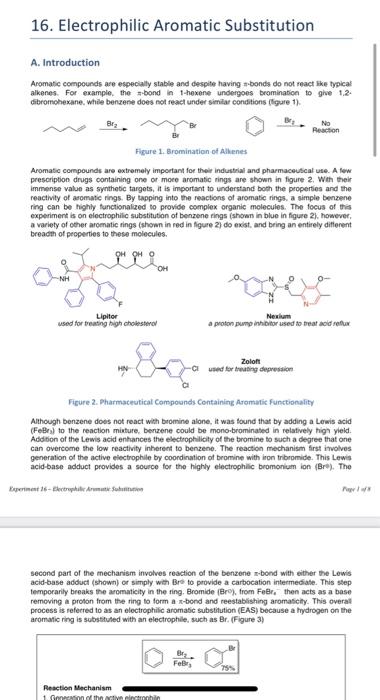
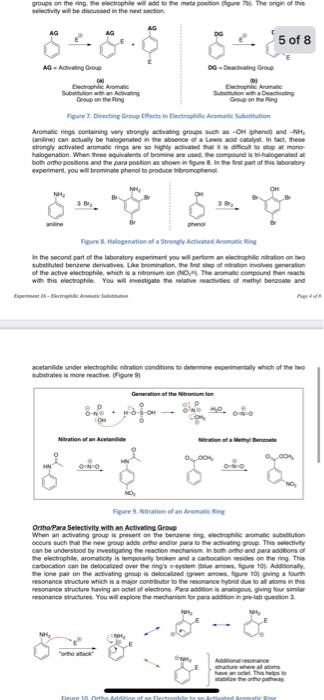
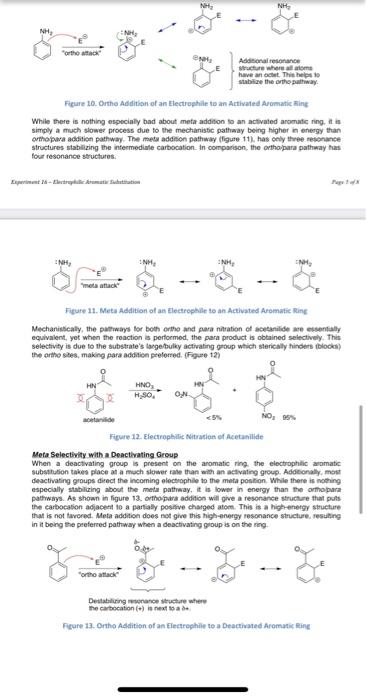
directing group. 3. The mechanism for the ortho substitution of aniline is shown in figure 10. Using acetanilide, which is an activating group like aniline, show the mechanism for para addition. Include all resonance structures. Explain why para addition, like ortho addition, is favored when an activating group is on the ring. 4. Based on the discussion of reactivity in the introduction, what do you hypothesize will be the major product of the competitive nitration reaction? 16. Electrophilic Aromatic Substitution A. Introduction Aromatic compeunds are especialy stable and despite having mibonds do not react the typicak alkenes. For example, the abond in thexene undergoes brominatice to give 1.2. dibromohexane. While bensene does not react under similar conditions (ligure 1). Figure 1. Bromination of Alkenes Aromate compounds are extremely important foe theit industeral and pharmaceuscal use. A few prescription drugs containing one or moee aromatic rings are shown in figure 2 . Wth their immense value as synthetic targets, it is important to understand both the properties and the reactivity of aromate rings. By tapping inte the reactions of aromatic rings. a simple benzene ring can be highly functionalzed to provide complex organic melecules. The focus of this experiment is on electrophilic substitution of benzene rings (shown in blue in figure 2), however, a variety of other aromatic rings (shown in red in figure 2) do exst, and bring an entirely different breadth of properties to these molecules. Ficure 2. Pharmateutical Compounds Containing Aromatic functionality Alhough benzene does not react with bromine alone, it was found that by adding a Lewis acid (FeBle) to the reaction miature. berzene could be mono-brominated in relafively high yieid. Addifion of the Lewis acid enhances the electrophilicily of the bromine to such a degree that one can overeome the low reactivity inherent to benzene. The reaction mechanism first itvolves generation of the active electrophile by coondination of bromine with iron tribromide. This Lewis acid-base adduct provides a source for the highly electrophilic bromonlum ion (Bri). The Paxt 1= second part of the mecharism involves reaction of the benzene -bond with either the Lewis acid-base adduct (shown) or simply with Bro to provide a carbocation intermedate. This slep temporarily breaks the aromaticity in the ting. Bnomide (Bro), from FeEr, then acts as a base removing a proton from the ring to form a -bond and reestablishing aromasctly. This overal process is refecred to as an electrophilic aromasic substitution (EAS) because a hydrogen on the aromatic ring is substiuled with an electrophile, such as Br. (Figure 3) second part of the mecharisen inotres maction of the berawe mbond with ether the Lewis acidbase adduct (showni) of simply whit Bev to provide a cabecilon intemediate. This steg process is referred to at an electrophils anmase mbethiten IEASI becisue a hytiogen on the Vigare 3. Elestropinlite Brumination of thenatint rings. Table 1 lests the five most comnos efectrophilc aromate sabuthison nactiont: Table 1. Typicat Condtions fle Renctrophilir Mrumatic Sibut mution Disubstituted Berrene Terminology The sermi arthe mete and para are trquerey uted be dewerto the locatoral iebationshio be ortho two substisents in a 1.3 relationship are said io be enefs, ane teo sabsibverts in at 1,4 relafionship are iaid to be pata. (Figure 4) Foyse 4, Drstit. Aleta, and Fara Termingiacy Directing Girbus Ellects thenelore reactivity of the aromatic ring. Some mbeituerts activite the ring making is more teactive than berafene ilone, while other matituerta deactive the ning mating if less meactive can be generalized in the folowing may: Actinating groups fopicaly contuin a bee gar on the atom that is diectly afachichine exsecten, however, and 5= x+5 groupe on the ing. He electephle wirl asd bs the mete pention (Hque fil The orign of the selectivay will be dicusted in the neat secton. Feure 7, Direnting Copug tffecti in fiectrephile Arsmpte Sutititution Aromatic rings containing very stroegly actialing groupe lises as Ort phenel and NH2 (miline) can actualy be hatogenated in me aboeson of a liss sod cacilat. In hact, these seingly activated aromate rings are so highly aftialed tie is a dificut to streg at mene: expeiment, you will berminate phenol to produce tibromephend in the second part of the laboralory experiment yoy will peform an elechophilc ntratoo on hoo subetinded berzese deriabives. Lae teominaton the fint ihe if aitatoe meshes pererafon with this electophile. You will inverigate the nelative mactives of mathyl benasase and acetarilide suder electrophilc ntation condtions to debemine especinentaly wheh of the two Mustrates is more mactien. (Figure 9 ? Fisure 9, Nhratien ef an leenuts hiss OrithoPare Selectivity with an Activeting Growe When an actiating proup is presert de the bertien ting ewelcephile armate aubuthich cecurs wuch hal me new group adds orte andior para is the activating prove. Tis selectivity ean be undestood by inestigating fer maction mecharisk in beth onto and para asdibons of The lone pai on the actiating grop is delocalted fgren anses. fege toi guing a foum tevonance thectures. You wit explote the michanivm lor pura adgbon in fot-lab quation 2. Fuggare 10. Ortho Addition of an Electrophine to an Activated Aromatic Aing While there is nothing especialy bad about mefar additon so an activated aromatic ring. it is simply a much slower process due to the mechanistic patheay being higher in energy than ovthorpura adtition pathway. Tha meda addisin pathway (figute 11), has enty three tesonahce structures stabilizing the infermediate cartbocation. In corncacison, the orthorpara pathway has forar resonance seructures. Firure 11. Meta Addition of an Electrophile to an Activated Aromatic Ring Mechanistically, the patiways for both ortho and para nitration of acetanilide are essentally. equivalent, yet when the reaction is performed, the para product is obbained selectively. Thes selectivity is due to the bubstrate's laggetbulky actirating group which sterically hindees iblockili) the tarethe sibes, making para addition preforred. (figese 127 Fifure 12. Efectroptilic Nitration of Acetanillale Mefa Selectivity with Deactrating Group When a deactivating group is present on the aromatic ring. the electrophilic aramatic substhytion takes place at a much slower rase than aith an activating group. Addeionally. mest deactivating groups direct the incoming electrophile to the mefa position. While there is nothing especially stabiluing about the mein pattraay, it is lower in energy than the orthoparim pathwilys. As shown in figure 13. orthopari adidicn will give a resorance struchure that pals. the carteceation aciacent 10 a parlaly positive charged atom. This is a high-energy struchure that is not tavored. Meta additige does not ghe this high-energy resonance structure. resuthrg in if being the prefereed pathway when a deactivating gioup is on the ming