Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answer question A by making a PT diagram; I will thumbs down any response without a PT diagram. Dandekar describes drawbacks of the ideal
Please answer question A by making a PT diagram; I will thumbs down any response without a PT diagram.
Dandekar describes drawbacks of the ideal mixture principle, and states that values cannot be estimated by using the ideal mixture principle for temperatures above the critical temperature of the most volatile component of the mixture. However, this statement is not entirely correct.
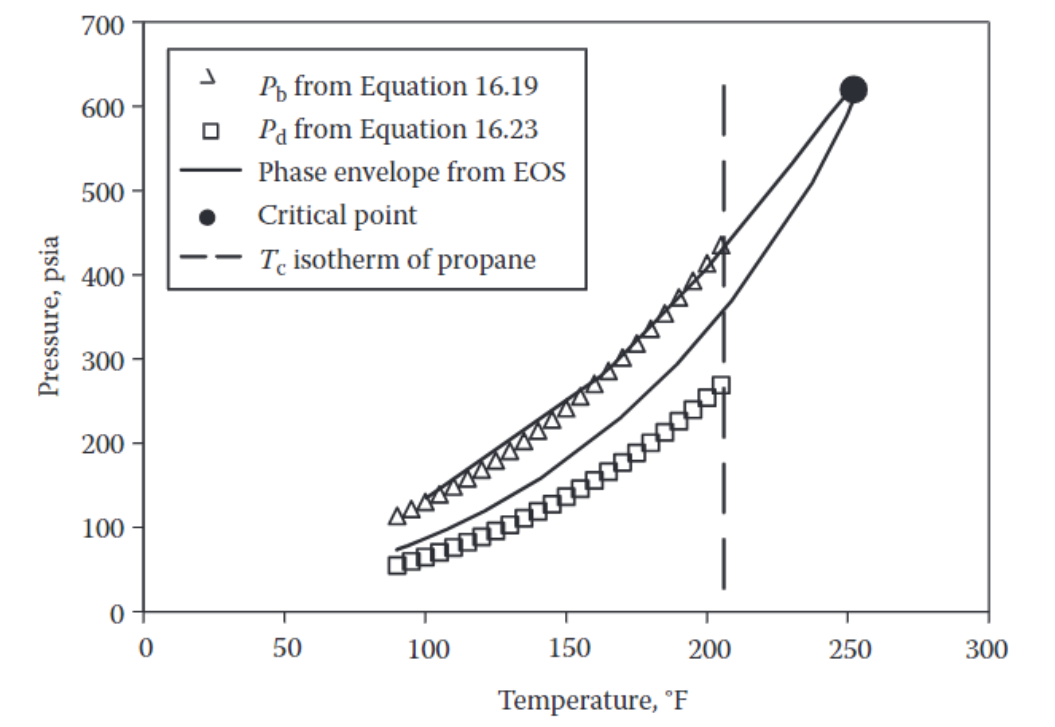
A Please disprove the above statement of Dandekar by using Equation given below for plotting the bubble and dew points for the mixture used in Figure also below from
to That is please make a PT diagram that shows and points based on Equation for the temperature range.
Pd
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


